Here's how much money Pfizer, Moderna and Johnson & Johnson could make from COVID vaccines
US has surpassed 29 million COVID-19 cases, 525,000 related deaths, according to Johns Hopkins University
Over 92 million COVID-19 vaccine doses have been administered across the United States as of Monday, according to the latest data from the Centers for Disease Control and Prevention.
There are currently three vaccines approved by the CDC and the Food and Drug Administration for emergency use, which were manufactured by Pfizer and BioNTech, Moderna, and Johnson & Johnson.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| PFE | PFIZER INC. | 27.05 | -0.17 | -0.62% |
| BNTX | BIONTECH SE | 110.34 | +3.72 | +3.49% |
| MRNA | MODERNA INC. | 41.95 | +0.94 | +2.29% |
| JNJ | JOHNSON & JOHNSON | 238.64 | -1.35 | -0.56% |
Here's a FOX Business roundup of the profit each company's vaccine is expected to bring in:
DEMOCRATS' COVID RELIEF BY-THE-NUMBERS BREAKDOWN IS JAW-DROPPING
Pfizer and BioNTech
Pfizer and BioNTech's Comirnaty vaccine, the first to receive an emergency use authorization from the FDA in December after it was found to be 95% effective, currently accounts for more than 46.8 million of the total vaccine doses administered in the United States, according to the CDC.
Pfizer's latest earnings report notes that Comirnaty profits are split 50-50 with BioNTech. The companies expect the vaccine to bring in revenues of approximately $15 billion in 2021. Pfizer and BioNTech plan to supply up to 1.3 billion doses of the vaccine worldwide by the end of 2021. The vaccine, which requires two doses, costs $39 in the United States and £28 in the EU.

Vials of Pfizer-BioNTech COVID-19 vaccines are ready to be injected to medical staff at the Ichilov Hospital in Tel Aviv, Israel, Sunday, Dec. 20, 2020. (AP Photo/Ariel Schalit)
Following Comirnaty's approval, the United States agreed to pay the companies $1.95 billion upon the receipt of the first 100 million doses. The agreement also allowed the federal government to purchase an additional 50 million doses. Later that month, Pfizer, BioNTech and the federal government reached a second agreement for 100 million more doses for the same $1.95 billion price tag. Under the second agreement, the companies will deliver at least 70 million of the additional doses by June 30, with the remaining 30 million doses to be delivered no later than July 31. The government also has the option to acquire up to an additional 400 million doses.
Pfizer and BioNTech also announced an agreement in November with the European Commission for 200 million doses of the vaccine to be distributed to the European Union's 27 member countries, with the option to request an additional 100 million doses. The European Comission made that request in December, Pfizer said.
Last month, Pfizer said the European Commission had reached a second agreement for an additional 200 million doses, 75 million of which will be supplied in the second quarter of 2021. The total number of doses to be delivered to the E.U. member states by the end of 2021 is now 500 million, with the potential to increase to 600 million based on the option granted in the new agreement.
MARRIOTT PAYING EMPLOYEES TO GET COVID-19 VACCINE
Moderna
Moderna's mRNA-1273 vaccine, the second to be approved for emergency use by the FDA after it was found to be 94.5% effective, accounts for more than 44.9 million of the total vaccine doses administered in the United States, according to the CDC.
The company noted in its fourth quarter earnings report that it expects $18.4 billion in anticipated product sales related to advanced purchased agreements for its vaccine. Moderna remains on track to deliver the first 100 million doses in the first quarter of 2021 and the second order of 100 million doses in May 2021. The company is aiming to build supply up to 1 billion doses in 2021, and 1.4 billion doses in 2022.
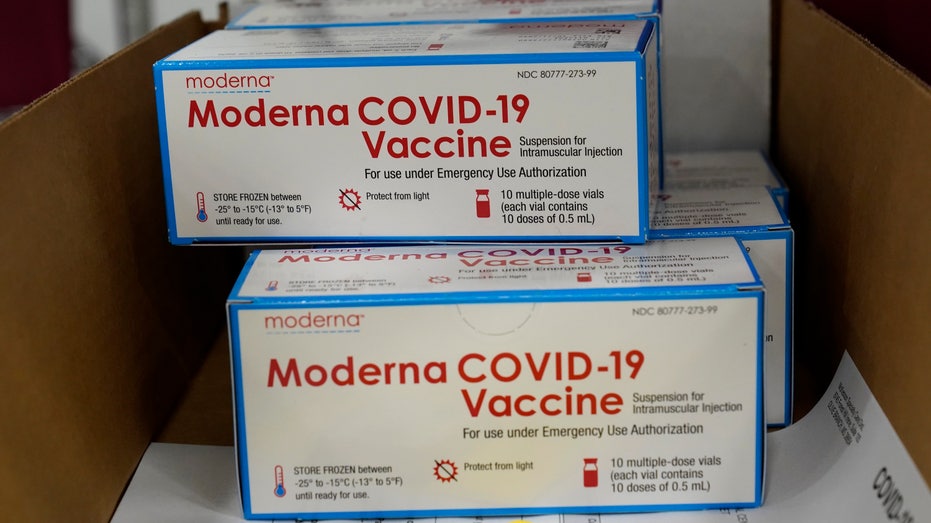
Boxes containing the Moderna COVID-19 vaccine are prepared to be shipped at the McKesson distribution center in Olive Branch, Miss., Sunday, Dec. 20, 2020. (AP Photo/Paul Sancya, Pool)
Moderna has reached an agreement with the United States for 300 million doses, with the option to purchase an additional 200 million doses. In addition, the company reached agreements with the European Union (310 million doses with option to purchase an additional 150 million doses in 2022), Japan (50 million doses), Canada (44 million doses), the Republic of Korea (40 million doses), the United Kingdom (17 million doses), Switzerland (13.5 million doses), Colombia (10 million doses), Israel (6 million doses) and Taiwan (5 million doses).
Moderna charges $30 for the required two shots in the U.S. and $36 in the E.U.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Johnson & Johnson's vaccine, manufactured by its pharmaceutical subsidiary Janssen, was given an emergency use authorization by the FDA on Feb. 27 and an official stamp of approval from the CDC a day later.
According to an analysis by the FDA, the Janssen vaccine was about 67% effective in preventing moderate to severe cases within 14 days after dosing and 66% effective against severe to critical cases after 28 days. In addition, the vaccine was approximately 77% effective in preventing severe or critical COVID-19 occurring at least 14 days after vaccination and 85% effective in preventing severe or critical COVID-19 occurring at least 28 days after vaccination.
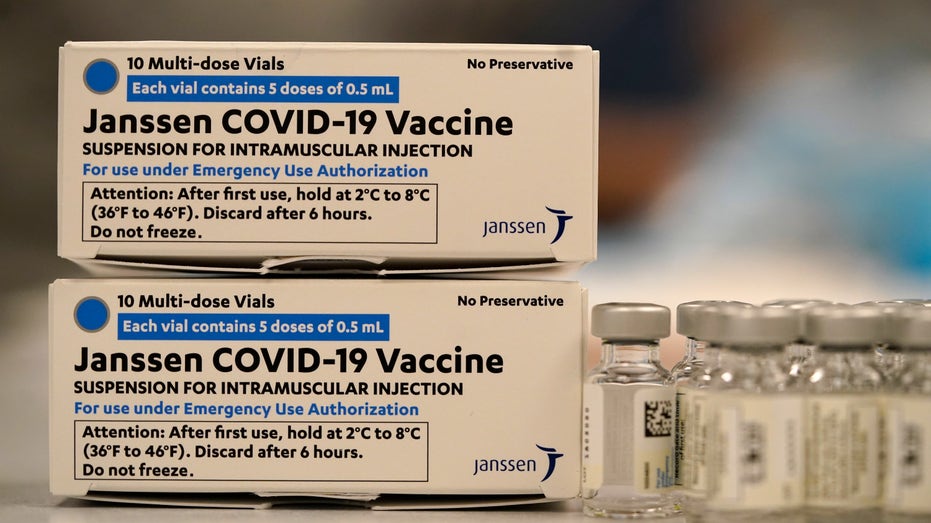
Boxes containing the Johnson & Johnson COVID-19 vaccine sit next to vials in the pharmacy of National Jewish Hospital for distribution early Saturday, March 6, 2021, in east Denver. Volunteers worked with nurses and physicians from National Jewis
The company is currently working to deliver its initial supply of 3.9 million vaccine doses to state health departments, pharmacies, federally qualified health centers and community vaccination centers across the country. Johnson & Johnson expects to deliver enough single-shot vaccines by the end of March to vaccinate more than 20 million Americans, and 100 million single-shot vaccines by June. Unlike the other two available vaccines, the Janssen vaccine requires a single dose.
The company is on track to produce 1 billion doses for global distribution by the end of 2021, which would generate up to $10 billion. In August, Johnson & Johnson reached a more than $1 billion agreement with the United States for 100 million doses of its vaccine, with the option to purchase an additional 200 million doses, for $10 per shot. In addition, Johnson & Johnson reached an agreement with the E.U. for up to 400 million doses.
CLICK HERE TO READ MORE ON FOX BUSINESS
According to Johns Hopkins University, the United States has surpassed 29 million COVID-19 cases since the pandemic began in March 2020, with over 525,000 related deaths.