Fat burner, growth hormone drugs recalled over product sterility concerns
Innoveix Pharmaceuticals, Inc. reported no serious side effects relating to the recall as of July 13
The Food and Drug Administration (FDA) has announced Innoveix Pharmaceuticals' voluntary recall of two medications over product sterility concerns.
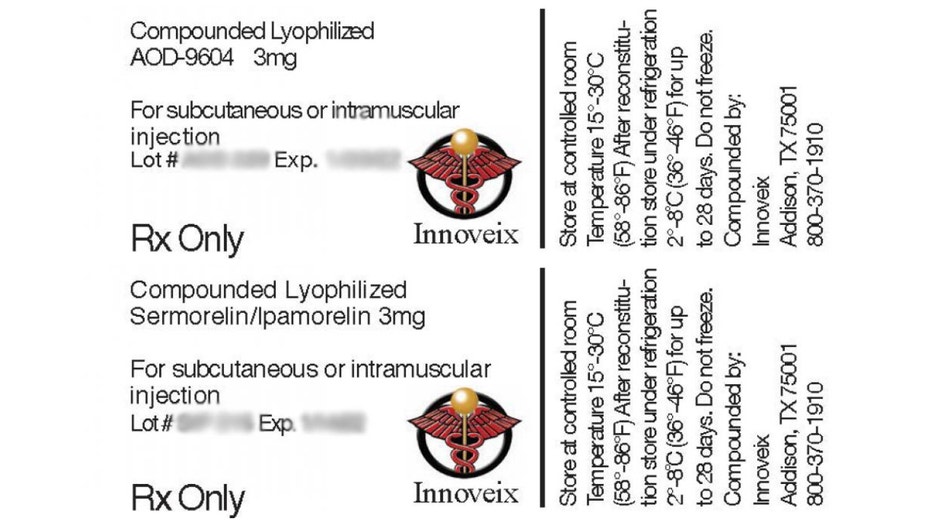
The prescribed injection drugs under the recall involve Sermorelin/ Ipamorelin 3mg and AOD-9604 3mg, meant to stimulate the release of growth hormones and help fight obesity through metabolism regulation. Consumers were advised not to use the products and seek a full refund.
The Food and Drug Administration (FDA) has announced Innoveix Pharmaceuticals' voluntary recall of two medications over product sterility concerns. (FDA)
JOHNSON & JOHNSON RECALLS SEVERAL SUNSCREENS: HOW TO CHOOSE THE RIGHT PRODUCT
"Administration of a drug product intended to be sterile, that is not sterile, could result in serious infections which may be life-threatening," reads the FDA’s recall notice posted July 13. "To date, Innoveix Pharmaceuticals, Inc. has not received any reports of adverse events related to this recall."
The FDA noted the recall was done out of "an abundance of caution and to promote patient safety." The pharmacy contacted potentially affected customers, the FDA said.
CLICK HERE TO KEEP READING FOX BUSINESS
The medications were distributed nationwide to customers and medical facilities in glass vials in a small 3 inch by 3 inch white box, according to the notice. Lots subject to the recall include Sermorelin / Ipamorelin 3mg Lot# SIP210; Exp: 12/15/2021; Lot# SIP215; Exp: 01/14/2022; Lot# SIP220; Exp: 01/23/2022 and AOD-9604 3mg Lot# AOD205; Exp: 11/09/2021; Lot# AOD210; Exp: 11/18/2021; Lot# AOD 215; Exp: 12/15/2021 and Lot# AOD202; Exp: 11/09/2021.
Consumers with questions or concerns can reach Innoveix Pharmaceuticals, Inc. by phone 800-370-1910 or innoveix@gmail.com.




















