At-home COVID-19 tests recalled after being illegally imported to US
The FDA did not authorize the tests for distribution or use in the US
Fox Business Flash top headlines for February 10
Check out what's clicking on FoxBusiness.com.
Certain at-home coronavirus tests are being recalled because officials discovered that they were illegally imported into the United States.
SD Biosensor Inc. issued the recall for its Standard Q COVID-19 Ag Home Test "due to confirmed reports that the test kits were illegally imported," according to a recall notice posted by the Food and Drug Administration (FDA).
CLICK HERE TO READ MORE ON FOX BUSINESS
The FDA did not authorize the tests for distribution or use in the U.S., the notice continued.
The company issued the recall out of an abundance of caution even though "there is no known distribution of these tests directly to consumers."
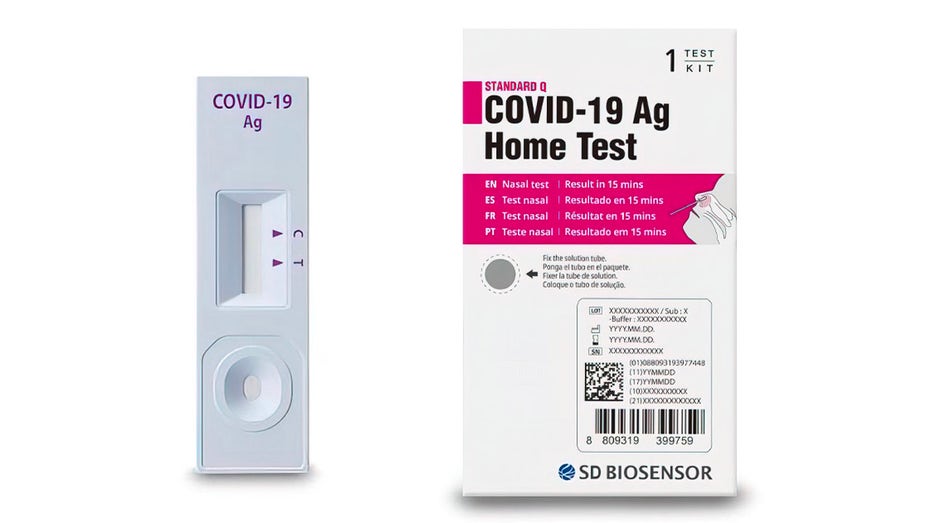
DD Biosensor, Inc., a global in-vitro diagnostics company, is voluntarily recalling its Standard Q COVID-19 Ag Home Test in the United States, due to confirmed reports that the test kits were illegally imported into the United States. (FDA)
However, in the event that consumers are in possession of the test, officials say they should discard it. If a consumer used the test, they are "encouraged to consider retesting with an FDA authorized or cleared test," according to the recall.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
The tests were only designed as an initial screening test result and "should not be the sole basis for the diagnosis" the notice continued, adding that another test is required for proper diagnosis.
The company says it "considers illegal importation to be a grave matter" and is taking measures to prevent any further attempts to illegally import the unauthorized tests.
In doing so, the company launched an investigation into how the product was illegally imported in the first place.
"Distributors or individuals who illegally imported the products initially sold outside the United States will be ordered to stop the illegal activity and initiate an immediate product recall," the notice said.





















