Companies testing coronavirus vaccines: Johnson & Johnson, Sanofi and more
Moderna, CanSino, Inovioa and Arcturus are also in the mix
Get all the latest news on coronavirus and more delivered daily to your inbox. Sign up here.
Johnson & Johnson is the latest company to tout its progress toward a usable coronavirus vaccine as competitors from the U.S. and abroad race to develop a way to prevent the virus that has killed at least 33,000 people worldwide.
Developing such a vaccine could cost well over a billion dollars and take years, although these companies say they're working on the quickest possible timeframe, vaccine policy expert Kelly Cappio of Avalere Health told FOX Business.
CORONAVIRUS VACCINE DEVELOPMENT COULD COST $1 BILLION
"Vaccine development in general involves ... a high risk of failure. These vaccines can't be developed overnight," Cappio said. "Obviously in an outbreak like this, the timeline is compressed because of an urgent public health need."
Johnson & Johnson
Johnson & Johnson's pharmaceutical company Janssen worked with Beth Israel Deaconess Medical Center, part of Harvard Medical School, to test and select its lead coronavirus vaccine candidate, the company said Monday.

A paramedic transports a patient into the Trauma Center at the Elmhurst Hospital Center, Sunday, March 29, 2020, in the Queens borough of New York. (AP Photo/Mary Altaffer)
Johnson & Johnson plans to move to human clinical studies by September and achieve emergency use authorization by early 2021.
The company has partnered with the U.S. Biomedical Advanced Research and Development Authority, which is part of the Department of Health and Human Services, to commit to investing more than $1 billion in fighting the coronavirus.
Moderna Therapeutics
Massachusetts-based Moderna Therapeutics was the first U.S. company to get a coronavirus vaccine ready for human testing. Its CEO said the company would put the vaccine at the same price point as similar existing vaccines.
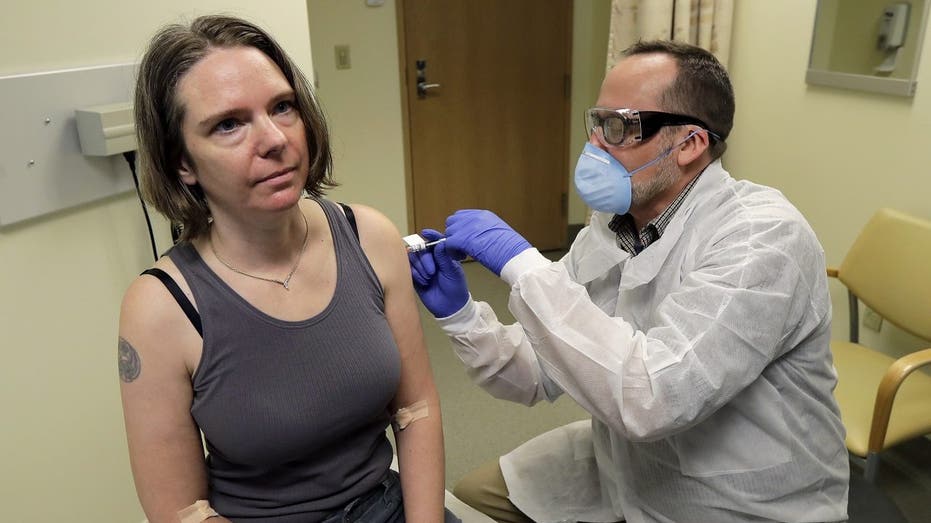
A pharmacist gives Jennifer Haller, left, the first shot in the first-stage safety study clinical trial of a potential vaccine for COVID-19, the disease caused by the new coronavirus, Monday, March 16, 2020, at the Kaiser Permanente Washington Health
The National Institutes of Health began a Phase 1 clinical trial of Moderna's investigational vaccine at Kaiser Permanente Washington Health Research Institute in Seattle in mid-March.
CanSino Biotechnology Inc.
China-based CanSino has gotten the go-ahead to begin human clinical testing in China. CanSino is known for creating the first Ebola vaccine to gain approval anywhere in the world.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| JNJ | JOHNSON & JOHNSON | 239.99 | +2.20 | +0.93% |
| MRNA | MODERNA INC. | 41.01 | +0.14 | +0.34% |
| SNY | SANOFI | 47.83 | +0.34 | +0.72% |
| INO | INOVIO PHARMACEUTICALS | 1.66 | +0.10 | +6.41% |
Inovio Pharmaceuticals
American biotech company Inovio Pharmaceuticals says it created a coronavirus vaccine three hours after getting access to the virus's genetic sequence in mid-January.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
"We were able to rapidly construct our vaccine in a matter of about three hours once we had the DNA sequence from the virus available because of the power of our DNA medicine platform," Dr. J. Joseph Kim, Inovio's president and CEO, told FOX Business in February.
Inovio is partnering with Ology Bioservices Inc. to manufacture vaccines for clinical trial thanks to a $11.9 million grant from the Department of Defense.
Arcturus Therapeutics
San Diego-based Arcturus Therapeutics said last week that it is evaluating its vaccine candidate but has not reached human trials. The company is working with Duke University School of Medicine and the National University of Singapore.
CLICK HERE TO READ MORE ON FOX BUSINESS
Sanofi
French company Sanofi is pursuing treatment and a vaccine for the deadly virus. Sanofi and U.S. company Regeneron have partnered to test Kevzara, a treatment for patients hospitalized with coronavirus.





















