Hospitals oppose positive COVID-19 test requirement for more Medicare funding
Trump administration’s condition for receiving enhanced Medicare funding aims to prevent fraud, but hospitals point to coronavirus testing problems
Many health-care companies and hospital industry groups are fighting a Trump administration policy tying extra federal coronavirus reimbursements to test results proving that patients are positive for Covid-19, saying the requirement unfairly deprives them of relief money established by Congress.
Legislation in March provided hospitals a 20% boost to the standard federal Medicare reimbursement for each patient admitted for coronavirus.
But the Centers for Medicare and Medicaid Services added a requirement, which took effect Sept. 1: For hospitals to receive the funding, each patient must have a documented positive Covid-19 lab test.
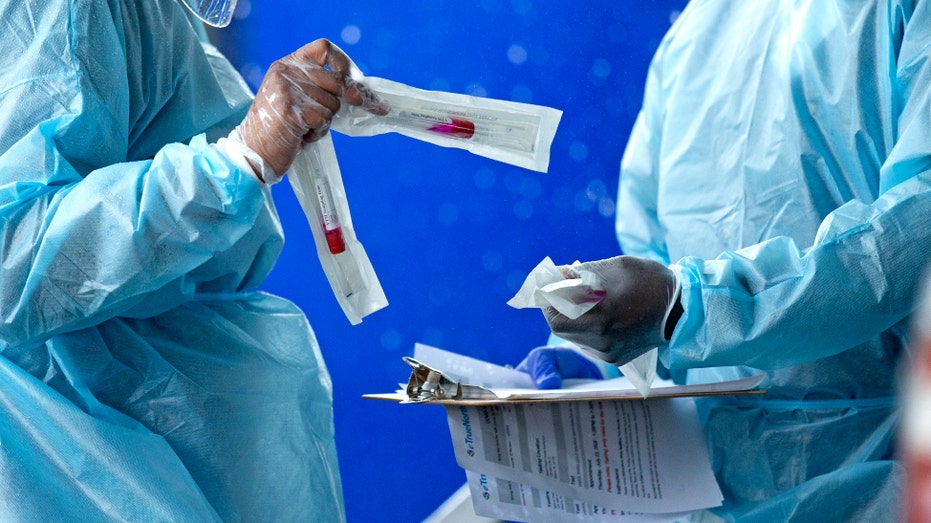
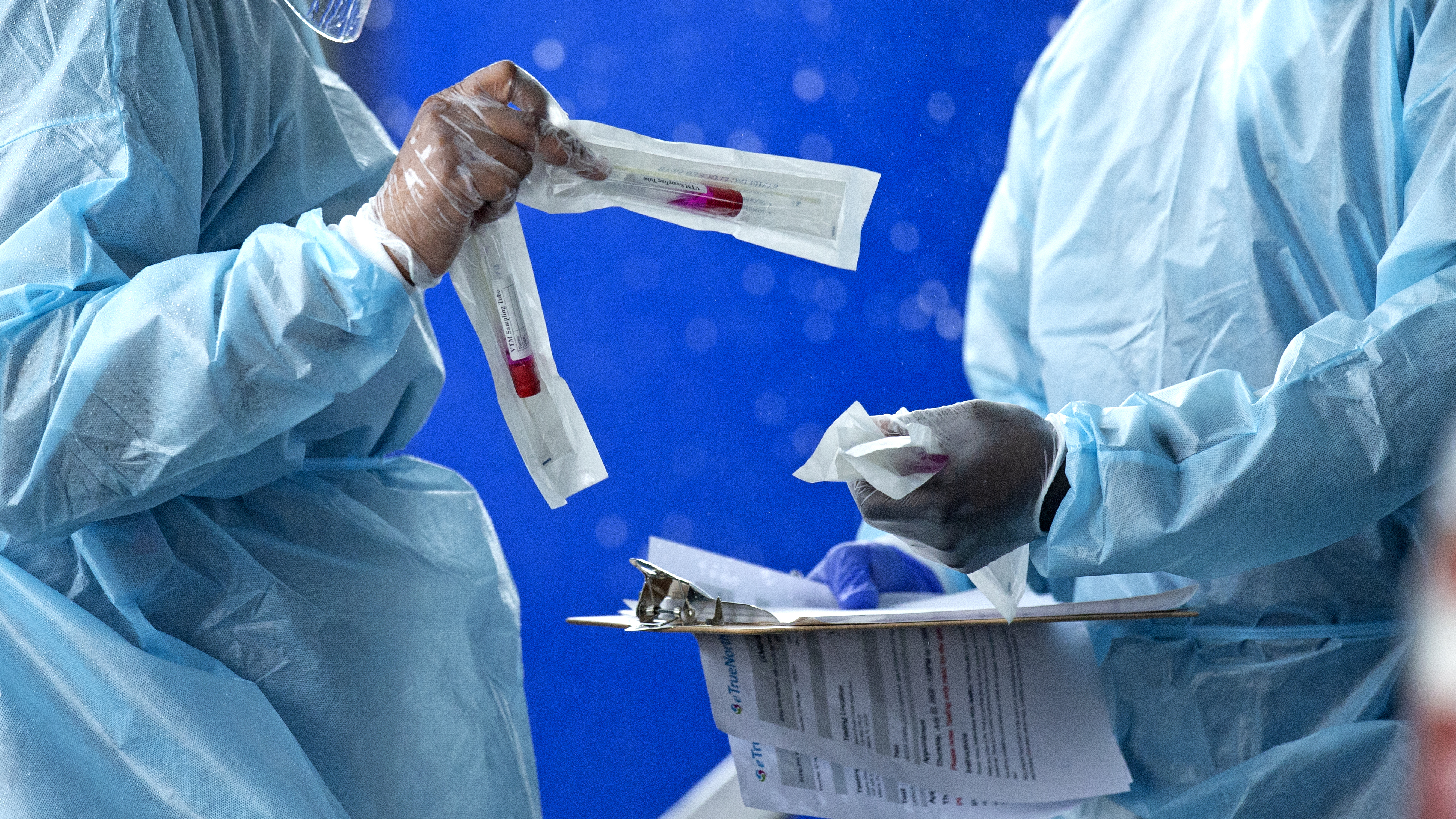
Health care workers prepare a COVID-19 test sample. (David Santiago/Miami Herald via AP)
7 CHARGED WITH LAUNDERING $2.1M, INCLUDING CORONAVIRUS RELIEF FUNDS
CMS officials said the requirement was added to protect against fraud since the funding was increased. "As part of Medicare's longstanding standard payment policies, Medicare providers are required to accurately document and bill for services provided based on a beneficiary's diagnosis," a spokesman said.
CMS is concerned that without a lab test showing someone has Covid-19, hospitals may code them incorrectly as having the virus and erroneously receive the 20% add-on. The agency said that it will review patient records after payments are made to confirm positive test results, and those lacking results would see their payments accounted for as overpayments.
Evidence suggests that hospitals could be overreporting Covid-19-related deaths for patients with certain medical conditions, such as end-stage renal disease and chronic kidney disease, according to an Aug. 20 report to CMS by Acumen, a Burlingame, Calif., provider of data analysis to government agencies.
Industry groups including the American Hospital Association, which represents nearly 5,000 hospitals and health-care providers, argue that if anything, many Covid-19 cases go undiagnosed. They said the new requirement is significant because tests can sometimes yield false negatives, and some people with Covid-19 don't test positive if the exam is conducted 14 days after infection.
False negative rates for PCR or molecular tests -- the most widely available type of test, which searches for the virus's genetic material in a nasal swab or saliva sample -- range from 2% to as high as 37%. False positives are generally around 5% or lower, but the accuracy remains uncertain and the results vary.
CMS officials said the requirement was added to protect against fraud since the funding was increased. (iStock)
MERCK COVID-19 VACCINE BEGINS HUMAN TESTING
Patients with long-term coronavirus health problems might not have a positive Covid-19 test because they were infected in February or March, when testing was restricted to people deemed at higher risk, including seniors, people who had been exposed to Covid-19 and medical staff. Hospital groups also said the policy unfairly overrides doctors' medical diagnoses and will result in unnecessary retesting at a time when U.S. testing capacity is already strained.
"It's unbelievably wasteful," said Brian Gragnolati, president and chief executive officer of Atlantic Health System, whose hospitals in New Jersey had as many as 900 Covid-19 patients in April. "This country has a testing problem. The time it takes to get a test varies widely, and some of the tests have a false negative. This seems unbelievably burdensome, and we're getting ready for another surge this fall."
Some Republicans have raised questions recently over whether the increased funding presents an incentive for doctors to inflate Covid-19 cases.
Hospital groups and doctors counter that they are being unfairly maligned as they stretch resources to grapple with the coronavirus, and say they are losing out on money. They also raise concerns about whether there has been enough oversight of federal stimulus funding and grants to providers. HHS is distributing $175 billion to hospitals and health-care providers on the front lines of the coronavirus response.
"They require you to jump through hoops to get paid, and it's a rigidity that's unfair," said Tom Nickels, executive vice president of government relations and public policy at the AHA.
The 20% add-on payment applies to patients who are admitted to hospitals for Covid-19 treatment and who are on Medicare, a federal health insurance program for people who are 65 and older or have specific disabilities. CMS in April had indicated that documentation from a doctor was all that was required for the funding boost. Under the new requirement, a lab test must be performed either before or during the admission.

Hospital groups and doctors counter that they are being unfairly maligned as they stretch resources to grapple with the coronavirus, and say they are losing out on money. (iStock)
GET FOX BUSINESS ON THE GO BY CLICKING HERE
The agency may conduct audits and reviews and recoup the add-on money if there is no positive test in the patient's medical record. CMS has said it would look at other medical factors in addition to a test if the test was done more than 14 days before admission, according to the agency guidance.
Some hospital leaders said they are concerned funding will be reduced during a time of historic financial pressures because of the pandemic. They also said the add-on payment still doesn't cover their costs for treating Covid-19 patients, because Medicare reimbursements trail private insurance reimbursement.
"This is not only unduly burdensome and potentially wasteful, but it also may lead to longer hospital stays, higher costs and additional discomfort for patients who are already suffering," according to an Aug. 26 letter to CMS from the hospital association.
Through May, there were a total of 101,348 discharges and total payments of $2.286 billion, for an average Medicare reimbursement of $22,556 per Covid-19 discharge, according to CMS data. For comparison purposes, the average Medicare spending in the first quarter of 2020 was $13,677 per discharge.
CMS is making other efforts aimed at ensuring accountability for taxpayer dollars, including a proposal for new reporting requirements in Medicaid, the federal-state program for low-income and disabled people. It also follows criticism from Democrats who say HHS hasn't provided enough oversight over coronavirus relief grants made to health-care providers. The HHS Office of Inspector General is auditing the distribution of grant funding.
Many patients hospitalized for Covid-19 are on Medicare because the virus has a more severe medical impact on seniors. People who are ages 65 to 74 are hospitalized at a rate five times higher than 18-to-29-year-olds, according to the Centers for Disease Control and Prevention.
Major for-profit hospital systems have seen higher profits since the pandemic began, but hospital groups said they are especially worried about the financial impact of the virus. The AHA in May estimated that the four-month financial impact of coronavirus was $200 billion in losses for hospitals and health systems. Hospitals had to pay for personal protective equipment and saw revenues drop because elective procedures were initially postponed because of the virus.