German biotech firm says its coronavirus vaccine will be ready for approval by December
1 billion doses could be produced by end of 2021
BERLIN—The German biotech firm that has partnered with Pfizer Inc. to develop a coronavirus vaccine is confident it will be ready to seek regulatory approval by the end of the year, according to its chief executive.
Several hundred million doses could be produced even before approval, and over 1 billion by the end of 2021, BioNTech SE co-founder and CEO Dr. Ugur Sahin told The Wall Street Journal.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| PFE | PFIZER INC. | 27.05 | -0.17 | -0.62% |
| BNTX | BIONTECH SE | 110.34 | +3.72 | +3.49% |
BioNTech, originally a cancer-treatment biotech company, is one of 17 firms world-wide that have started human trials on a vaccine against Covid-19. The company, based in the city of Mainz and listed on the Nasdaq stock exchange, was founded in 2008 by Dr. Sahin and his wife, Dr. Özlem Türeci, both the children of Turkish immigrants.
BIONTECH AND PFIZER'S CORONAVIRUS VACCINE SHOWS POTENTIAL IN HUMAN TRIAL
The vaccine BioNTech is developing uses experimental technology known as messenger RNA, or mRNA. Pending approval by authorities, BioNTech expects to begin the final stage of the testing process, known as Phase 3 trials, at the end of July. These would see 30,000 people take part in a randomized study of the vaccine that is expected to be completed by the end of the year, when the company would seek market approval from regulators across the globe.
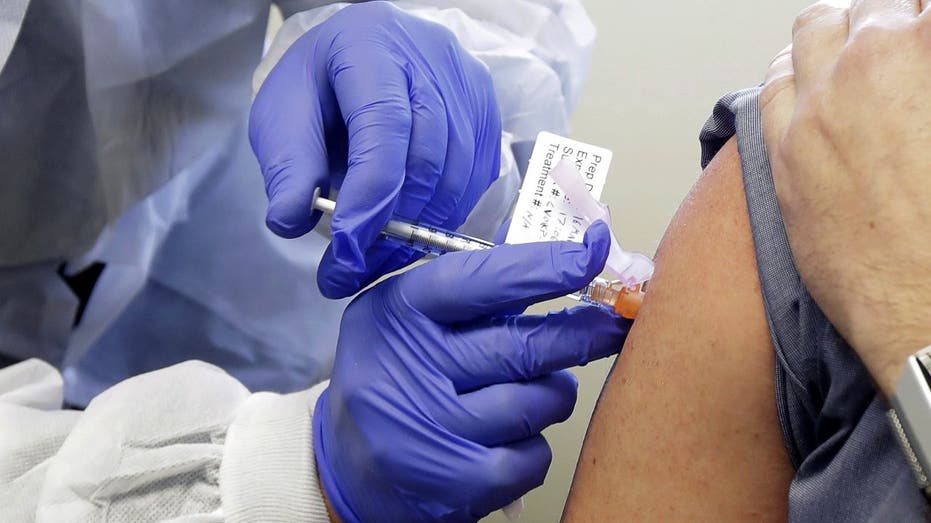
Neal Browning receives a shot in the first-stage safety study clinical trial of a potential vaccine for COVID-19. (AP Photo/Ted S. Warren)
The final stage of clinical trials is designed to test the efficacy of a vaccine in protecting from infection after the drug has been found to be safe in humans. Approval times could vary across jurisdictions, but BioNTech would begin vaccine production while awaiting such approvals.
BioNTech’s partnership with Pfizer in the U.S., as well as other partnerships with international companies such as China’s Shanghai Fosun Pharmaceutical Co. Ltd., would then enable the company to quickly produce vast amounts of vaccine doses for the global market.
WHICH COUNTRY WILL DEVELOP CORONAVIRUS VACCINE FIRST?
But even as BioNTech and its partners are already scaling up production capacities in anticipation of approval, the coronavirus has become so widespread—over 13 million people around the world had tested positive by Friday—that it would take about 10 years before humanity achieved sufficient immunity to the disease, even if several companies launch a vaccine at the same time, according to Dr. Sahin.
“I assume that we will only be done with this virus when more than 90% of the global population will get immunity, either through infection or through a vaccine,” he said in a telephone interview Wednesday night.
A researcher at Protein Sciences moves a vial in a lab, Thursday, March 12, 2020, in Meriden, Conn. (AP Photo/Jessica Hill)
BioNTech’s mRNA approach uses a genetic mechanism to induce the human body to produce certain proteins that generate antibodies and cellular immunity against the novel coronavirus.
If successful, these and comparable vaccines would be easier and cheaper to manufacture than traditional vaccines based on inactivated or partial virus cells. But no such vaccine has yet been approved for use outside clinical studies. A first batch of BioNTech’s vaccine would take between nine and 11 days to produce, the company said.
US TO ACCUSE CHINA OF ATTEMPTS TO HACK CORONAVIRUS RESEARCH
Pfizer owns about 1% of BioNTech and collaborates in developing the new vaccine, but all rights belong to the German company, which is doing the bulk of the research. BioNTech is majority-owned by the twin German investors Thomas and Andreas Strüngmann. Other shareholders include the Bill and Melinda Gates Foundation, Sanofi SA and Genentech, a unit of Roche Holding AG.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| SNY | SANOFI | 48.27 | +0.44 | +0.92% |
| RHHBY | ROCHE HOLDING AG | 57.11 | -0.59 | -1.02% |
Other companies already conducting human trials include Moderna Inc., which is also starting its advanced-stage trial this month. In August another vaccine co-developed by the University of Oxford and AstraZeneca PLC will also enter phase 3 trials, followed by a product developed by Johnson & Johnson in September.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| MRNA | MODERNA INC. | 41.95 | +0.94 | +2.29% |
| AZN | ASTRAZENECA PLC | 188.25 | -4.85 | -2.51% |
| JNJ | JOHNSON & JOHNSON | 238.64 | -1.35 | -0.56% |
BioNTech’s candidate showed better-than-expected results in the early-stage study that was published on July 1, the company said. Volunteer test subjects had higher levels of antibodies than convalescing Covid-19 patients four weeks after being vaccinated and seven days after getting a second dose.
The vaccine candidate also appeared not to cause any serious side effects, Dr. Sahin said.
“Our early data so far has shown that our vaccine candidate can produce antibodies at a higher level than those found in people who have recovered from a Covid-19 infection,” Dr. Sahin said.
NOVOVAX GETS $1.6B FROM GOVERNMENT TO DEVELOP CORONAVIRUS VACCINE
The study data was published online and submitted to a medical journal but hasn’t yet been peer-reviewed.
If and when the vaccine is approved, mass vaccinations could start immediately in the U.S., Europe and elsewhere, Dr. Sahin said. The effort would however depend on countries’ logistical capacities as well as popular acceptance.
Drs. Sahin and Türeci have become leaders in Germany’s biotech industry after each of their families emigrated from Turkey in search of employment, with Dr. Sahin’s parents having worked at a Ford Motor Co. plant.

A laboratory technician places various blood vial samples into a rack that is sitting on a desk. (iStock)
In addition to serving as BioNTech’s CEO, Dr. Sahin is a professor of experimental oncology at the University of Mainz. Dr. Türeci is chief medical officer of the company, which now employs around 1,400 people and had a market value of $15 billion on Wednesday. She previously served as CEO of Ganymed Pharmaceuticals AG, the first biotech company founded by the couple, which they sold to Japanese pharmaceuticals firm Astellas Pharma Inc. for up to $1.4 billion in 2016.
BioNTech’s core research was focused on mRNA-based cancer treatments tailored to individual patients, but after reading reports about the novel virus in late January, Dr. Sahin says he decided the company should shift its focus to researching a coronavirus vaccine.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
At the time, few scientists and governments expected the pathogen to develop into today’s devastating pandemic. Some of Dr. Sahin’s researchers were skeptical about the decision to reconfigure the entire operation, but they became convinced as countries started going into coronavirus-induced lockdowns.
Dr. Sahin dismisses skepticism about the yet-untested mRNA technology and says that the progress made so far is unprecedented in the history of vaccine research.
“It is in the nature of innovation to take center stage at a time of great urgency,” Dr. Sahin said. “The world has never seen such a rapid global development of new vaccines, just as it has not seen such a quick, global spread of a pandemic before.”
CLICK HERE TO READ MORE ON FOX BUSINESS
Write to Bojan Pancevski at bojan.pancevski@wsj.com