Eli Lilly says FDA approval of Alzheimer’s drug donanemab could come later this year
FDA recently approved Alzheimer's treatment Leqembi
New drug could provide help for Alzheimer’s disease patients
Fox News chief Washington correspondent Mike Emanuel reports on the FDA postulating the approval of a new Alzheimer’s disease drug on ‘The Evening Edit.’
Drugmaker Eli Lilly & Co. said Monday that a second drug that modestly slows the progression of Alzheimer’s disease could be approved by the end of this year.
The manufacturer said donanemab significantly slowed cognitive and functional decline in people with early symptomatic Alzheimer's disease, with nearly half of the participants at an earlier stage of disease on the drug with no clinical progression after one year.
Eli Lilly previously announced that donanemab met the primary and all cognitive and functional secondary endpoints in the Phase 3 study.
Submission to the Food and Drug Administration for traditional approval was completed last quarter and submissions to other global regulators are currently ongoing.
ALZHEIMER'S DRUG LEQEMBI: WHAT TO KNOW

Eli Lilly headquarters in Indianapolis, Indiana, on Wednesday, May 3, 2023. (Photographer: AJ Mast/Bloomberg via Getty Images / AP Newsroom)
The company said the majority will be completed by year's end.
"If approved, we believe donanemab can provide clinically meaningful benefits for people with this disease and the possibility of completing their course of treatment as early as 6 months once their amyloid plaque is cleared. We must continue to remove any barriers in access to amyloid-targeting therapies and diagnostics in an already complex healthcare ecosystem for Alzheimer's disease," said Anne White, executive vice president of Eli Lilly.
The study – published by the Journal of the American Medical Association – found that donanemab slowed Alzheimer’s disease progression by 35% relative to placebo in a 19-month-long trial that included more than 1,700 early-stage participants ages 60-85.
Study participants at the earliest stage of the disease had an even greater benefit, and there was a 60% slowing of decline compared to placebo.

A sign for the Food And Drug Administration is seen outside of the headquarters on July 20, 2020, in White Oak, Maryland. ((Photo by Sarah Silbiger/Getty Images) / AP Newsroom)
GET FOX BUSINESS ON THE GO BY CLICKING HERE
If cleared, donanemab would be only the second Alzheimer’s treatment convincingly shown to delay the disease, following the FDA's approval of Biogen and Eisai’s Leqembi.
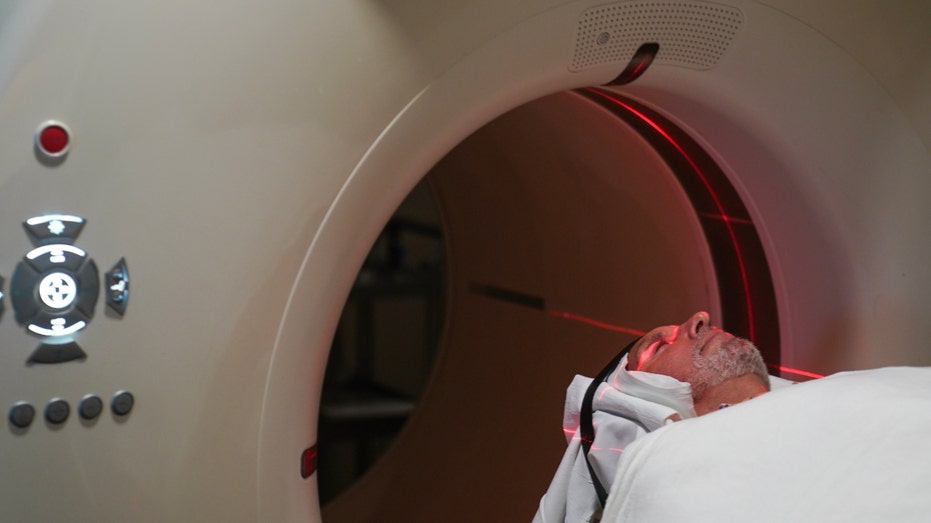
Jay Reinstein, who suffers from Alzheimer's, prepares to receive a PET scan at MedStar Georgetown University Hospital in Washington, D.C., on June 20, 2023. ((Photo by Michael Robinson Chávez/The Washington Post via Getty Images) / AP Newsroom)
Both donanemab and Leqembi are lab-made antibodies, administered intravenously, that target sticky amyloid buildup in the brain. Notably, the drugs also come with a serious safety concern – brain swelling or bleeding that, in the Lilly study, was linked to three deaths.
CLICK HERE TO READ MORE ON FOX BUSINESS
About a quarter of donanemab recipients showed evidence of brain swelling, and about 20% had microbleeds.
More than 90% of the study’s participants were White, leaving little data about how other populations might respond to the drug.
The Associated Press contributed to this report.





















