Pfizer expands smoking drug Chantix recall
The recall is due to presence of a nitrosamine, N-nitroso-varenicline, which may be linked to cancer
Fox Business Flash top headlines for September 16
Check out what's clicking on FoxBusiness.com.
Pfizer has expanded a voluntary recall of its anti-smoking treatment Chantix, covering all lots of the drug's 0.5 and 1 milligram tablets.
Pfizer has expanded a voluntary recall of its anti-smoking treatment Chantix, covering all lots of the drug's 0.5 and 1 milligram tablets.
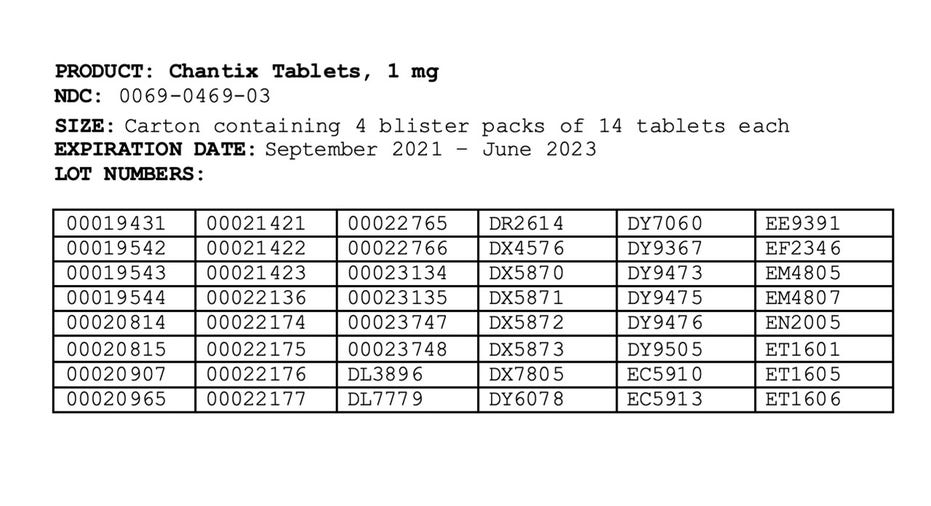
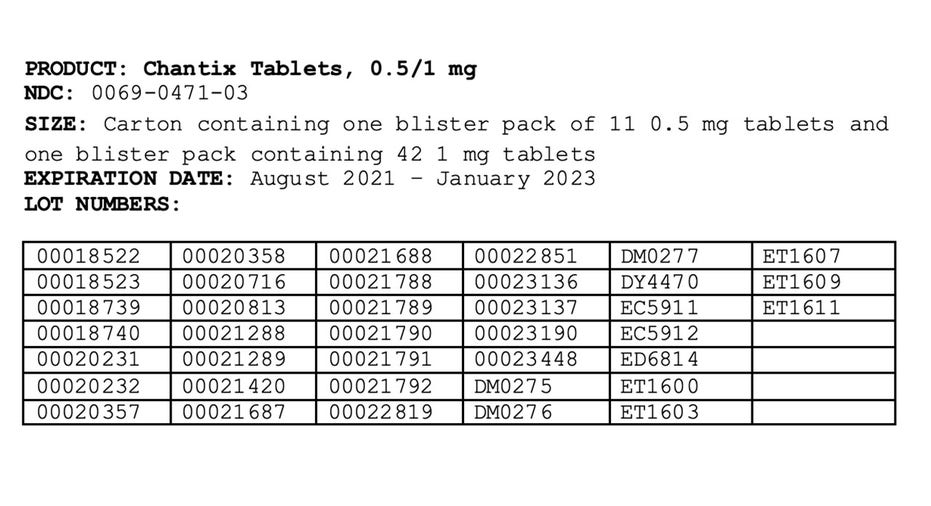
The affected products were distributed nationwide to wholesalers and distributors in the United States, US Virgin Islands and Puerto Rico between May 2019 and September 2021.
The affected products were distributed nationwide to wholesalers and distributors in the United States, US Virgin Islands and Puerto Rico between May 2019 and September 2021.
DESSERT ITEMS RECALLED OVER ALLERGY CONCERNS
The drug's recall, which was first issued in July, is due to the presence of a nitrosamine called N-nitroso-varenicline, which has been found to be at or above the Food and Drug Administration's interim acceptable intake limit.
Most consumers are exposed to nitrosamines to some degree, with the chemical being common in water and foods, including cured and grilled meats, dairy products and vegetables.
"Long-term ingestion of N-nitroso-varenicline may be associated with a theoretical potential increased cancer risk in humans, but there is no immediate risk to patients taking this medication," Pfizer said in its recall notice.
CLICK HERE TO READ MORE ON FOX BUSINESS
Wholesalers and distributors with an existing inventory of Chantix tablets are urged to stop use and distribution of the drug and quarantine the product immediately.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| PFE | PFIZER INC. | 27.02 | -0.19 | -0.69% |
Pfizer recommends that patients taking Chantix consult with their healthcare provider about alternative treatment options. In addition, patients with Chantix tablets should contact Stericycle Inc. at 888-276-6166 for instructions on how to return and obtain reimbursement for their product.
To date, Pfizer has not received any reports of adverse events related to the recall.