Twitter limits Donald Trump Jr.'s account features after hydroxychloroquine tweet
Post was 'in violation of our COVID-19 misinformation policy'
Twitter confirmed on Tuesday that it restricted some of Donald Trump Jr.'s account capabilities after he posted a video about hydroxychloroquine, a highly debated drug that his father, the U.S. president, has touted as a coronavirus treatment.
A Twitter spokesperson confirmed that Donald Trump Jr.'s account will have limited functionality for 12 hours, and the social media site deleted his tweet sharing a video that appeared to show doctors discussing the benefits of the drug for COVID-19 patients.
TWITTER DELETES VIDEO PROMOTED BY TRUMP ON HYDROXYCHLOROQUINE USE FOR CORONAVIRUS
The younger Trump's tweet "was in violation of our COVID-19 misinformation policy. We are taking action in line with our policy here," the spokesperson said.

(AP Photo/David J. Phillip, File)
Twitter's COVID-19 misinformation policy states that its teams and machine-learning tools are working to identify and label or remove any tweets that spread potentially harmful misinformation regarding COVID-19 "to keep people safe on Twitter."
CAN HYDROXYCHLROQUINE TREAT CORONAVIRUS?
"We’ll continue to prioritize removing content when it has a clear call to action that could directly pose a risk to people’s health or well-being," the company said in a March blog post.
While Twitter's automated misinformation efforts are meant to be consistent, "they can sometimes lack the context" that the company's human moderators have, which may result in the website "making mistakes," the company noted. Twitter is not permanently suspending any accounts "based solely on our automated enforcement systems" for that reason.
WHO PAUSES CORONAVIRUS HYDROXYCHLOROQUINE TRIAL OVER SAFETY CONCERNS
Hydroxychloroquine has not been proven so far to be an effective treatment for COVID-19, and research shows mixed results. Several studies, including one widely shared report from Oxford University, published on June 5, found no "beneficial effect" after using the drug on COVID-19 patients.
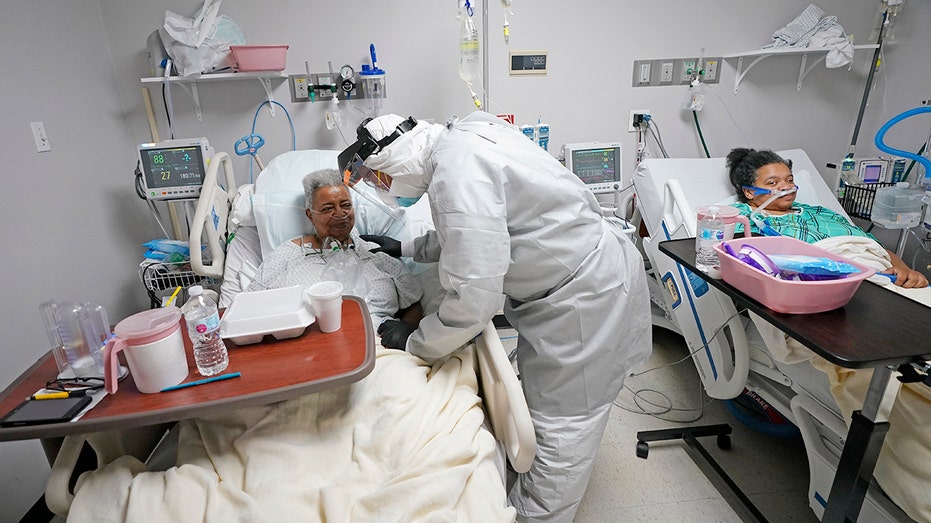
Dr. Joseph Varon, center, visits with Dorothy Webb, left, and her daughter, Tammie, while making his rounds inside the coronavirus unit at United Memorial Medical Center in Houston in early July. (AP Photo/David J. Phillip)
The Food and Drug Administration retracted its Emergency Use Authorization for the drug on June 15, and several organizations stopped clinical trials following the results of the Oxford study.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
A separate study from Henry Ford Hospital researchers, however, found COVID-19 patients treated with the drug, as well as those treated with a combination of hydroxychloroquine and an antibiotic, had higher survival rates than those who were not treated with hydroxychloroquine.
There is no known antiviral treatment for COVID-19 to date, according to the Centers for Disease Control and Prevention.
Side effects of hydroxychloroquine include an unstable heartbeat, low blood pressure and muscle or nerve damage.
CLICK HERE TO READ MORE ON FOX BUSINESS




















