Coronavirus vaccine candidates' pivotal US testing to start this summer
Moderna Inc.'s vaccine is set to be first
The federal government plans to fund and conduct the decisive studies of three experimental coronavirus vaccines starting this summer, according to a lead government vaccine researcher.
These phase 3 trials are expected to involve tens of thousands of subjects at dozens of sites around the U.S., John Mascola, director of the vaccine research center at the National Institute of Allergy and Infectious Diseases, said in an interview. Meant to determine a vaccine's safety and effectiveness, they would mark the final stage of testing.
ARE CORONAVIRUS TEMPERATURE CHECKS EFFECTIVE?
Moderna Inc.'s vaccine is set to be first, starting in July, followed in August by one co-developed by Oxford University and AstraZeneca PLC and in September by Johnson & Johnson's, he said.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| JNJ | JOHNSON & JOHNSON | 240.00 | +2.26 | +0.95% |
| AZN | ASTRAZENECA PLC | 193.10 | +5.91 | +3.16% |
| MRNA | MODERNA INC. | 41.01 | +0.14 | +0.34% |
The timetable suggests researchers are making relatively rapid progress advancing their vaccines through earlier stages of testing--focused on whether they are safe and induce the desired immune response--to at least merit the planning.
"We will want to use the investigative resources of the country as best we can to optimize us getting an answer as quickly as possible," said Larry Corey, a vaccine and infectious-disease specialist at the Fred Hutchinson Cancer Research Center in Seattle and member of a committee advising the National Institutes of Health on the design of the coronavirus-vaccine trials.
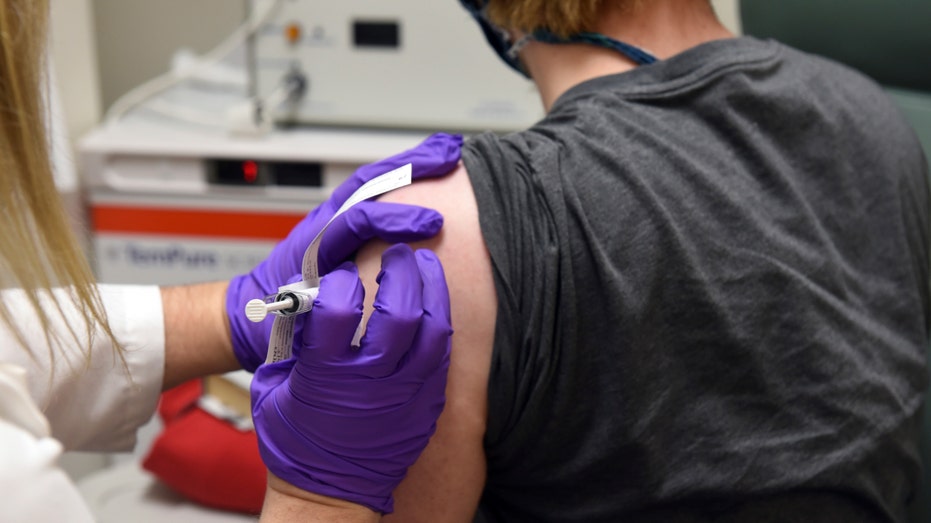
This May 4, 2020 photo from the University of Maryland School of Medicine, the first patient enrolled in Pfizer's COVID-19 coronavirus vaccine clinical trial at the University of Maryland School of Medicine in Baltimore, receives an injection. (Unive
A Moderna spokesman confirmed the plan for the NIH-funded trial of its vaccine. An AstraZeneca spokeswoman on Tuesday evening said the company's recent contract with a U.S. health agency would support a large study but couldn't confirm the start date. A J&J spokesman declined to comment.
WHO IS CDC DIRECTOR ROBERT REDFIELD?
Separately, Pfizer Inc., which with partner BioNTech SE has one of the more advanced vaccine candidates, could begin its phase 3 trial as early as July, said a person familiar with the matter. Pfizer isn't participating in the NIH testing program.
Many vaccine candidates fail to make it through the trials.
"There's a lot of optimism in our community that a vaccine should be possible, but we are very focused on the fact that that has to be proven in clinical trials," Dr. Mascola said in an online video discussion hosted by the Fred Hutchinson Cancer Research Center on Friday.
There aren't any vaccines proven to work against the new coronavirus. Public-health authorities say one or more will be needed before life can fully return to normal, because unlike drugs, vaccines could prevent infections or at least prevent or limit the severity of Covid-19, the disease caused by the coronavirus.
An existing network of researchers who have run HIV vaccine studies, which Dr. Corey leads, will help to conduct the large coronavirus vaccine studies, he said.
The studies will be designed, Dr. Corey said, to show more definitively whether the vaccines safely prevent disease. Researchers would start the phase 3, NIH-funded studies only once they have sufficient evidence from the earlier testing that the vaccines are safe and elicit certain immune responses in people, Dr. Corey said. The studies are also subject to clearance by regulators.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Each vaccine study is expected to include roughly 30,000 people, some receiving the experimental shots and others placebo shots, though the final number of participants could vary, Dr. Mascola said. Each study will be conducted at more than 50 sites, primarily in the U.S. but possibly including other countries, Dr. Corey said.
Researchers will track whether people who receive the vaccines are infected with the virus and develop Covid-19 at lower rates than those who get placebo. The studies will be conducted in locations where the virus continues to spread, Dr. Corey said.
The government may plan similarly large trials for additional coronavirus vaccines in development, such as those being developed by Sanofi SA and Merck & Co. Dr. Corey said testing plans for other vaccines haven't been completed.
Last month, Moderna said its vaccine, co-designed with NIH researchers, was generally safe and induced immune responses in a small, early-stage study. The second phase of testing just began.
CLICK HERE TO READ MORE ON FOX BUSINESS
Human testing of the vaccine from AstraZeneca and Oxford's Jenner Institute has started, but results haven't been released. J&J said previously it would start human testing by September.
Researchers hope the trials will yield answers within six to eight months of their starts, Dr. Corey said. Some vaccine developers have said their shots could become available for emergency use on a shorter timeline, possibly as soon as the fall.
Though each vaccine will be tested in a separate trial, the companies are coordinating certain aspects, such as using the same independent committee to monitor safety, according to Dr. Corey. This will allow researchers to better compare how the vaccines perform, and whether certain shots are better suited to certain subpopulations, he said.