Why Regeneron Pharmaceuticals, Inc. Stock Slipped 32.4% in 2016
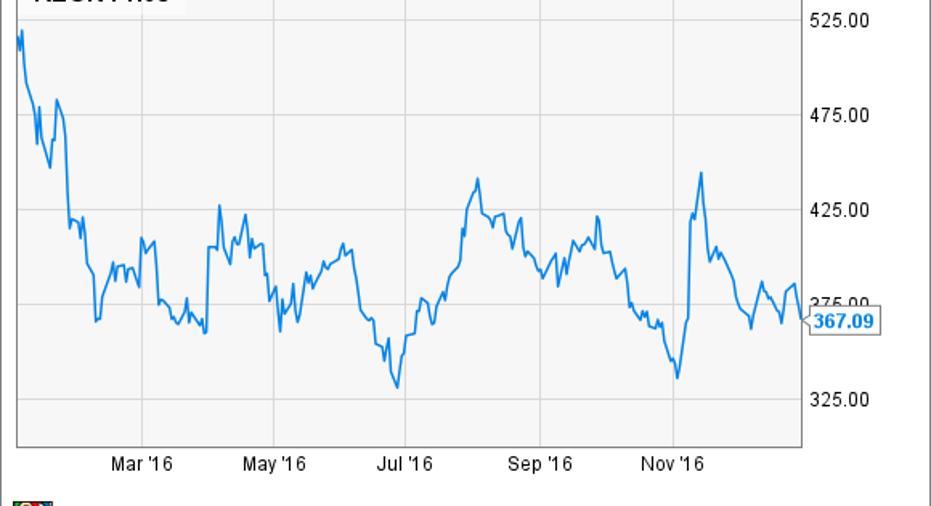
Image source: Getty Images.
What happened
Shares of Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN), a biopharmaceutical company, slid 32.4% last year, according todata from S&P Global Market Intelligence.A handful of issues concerning Sanofi (NYSE: SNY)partnered programs continue to weigh on the biotech stock.
So what
Regeneron's stock fell along with its biotech peers at the beginning of 2016, but it wasn't long before more specific issues that continue to hound the company and its big pharma partner reared their ugly head. In March, patent litigation concerning the partners' next-generation cholesterol-lowering drug, Praluent, drove down the stocks. Those worries took center stage more recently when a U.S. District Court issued an injunction that could halt U.S. Praluent sales 45 days from Jan. 5,2017, if the partners can't get an appellate court to suspend it first.
The next heaviest weight pushing down Regeneron stock was a surprising complete response letter (i.e. FDA rejection) for rheumatoid arthritis candidate sarilumab. The drug handily outperformed the world's best-selling drug, Humira, in a head-to-head trial, and was expected to begin earning a significant share of a rheumatoid arthritis space valued at around $18 billion annually.
Now what
Praluent is a highly effective cholesterol-reducing drug of the same class as Amgen's Repatha that was expected to reach peak annual sales topping $3 billion, but its launch has been disappointing so far. End payers are loathe to pay around $14,000 per year for the therapy without evidence it actually lowers risk of heart attack and stroke with long-term use. Regeneron and Sanofi have invested heavily in long-term outcome studies that will hopefully prove Praluent can provide a cardiovascular benefit that justifies its expense. Losing the ability to sell it throughout the U.S. would be a very expensive step backward.
Meanwhile, according to Regeneron and Sanofi, the Food and Drug Administration refused to approve the sarilumab application without more information regarding the drug's manufacturing process. The FDA has been issuing complete response letters citing manufacturing concerns with alarming frequency lately. Manufacturing concerns are easier to fix than safety or efficacy issues, but they can still delay potential launches for around a year.
The clouds could begin to clear for Regeneron and Sanofi this March when the FDA is expected to make a decision concerning an eczema candidate, Dupixent, under review. There is a dearth of effective treatments for the fairly large population of moderate to severe eczema patients, and Dupixent could generate annual sales of more than $4 billion. Investors will want to keep their fingers crossed that the partners will finally receive a bit of good news and enjoy a successful new drug launch.
10 stocks we like better than Regeneron Pharmaceuticals When investing geniuses David and Tom Gardner have a stock tip, it can pay to listen. After all, the newsletter they have run for over a decade, Motley Fool Stock Advisor, has tripled the market.*
David and Tom just revealed what they believe are the 10 best stocks for investors to buy right now... and Regeneron Pharmaceuticals wasn't one of them! That's right -- they think these 10 stocks are even better buys.
Click here to learn about these picks!
*Stock Advisor returns as of January 4, 2017
Cory Renauer has no position in any stocks mentioned. You can follow Cory on Twitter @coryrenauer or LinkedIn for more biopharma investing insight.The Motley Fool has no position in any of the stocks mentioned. The Motley Fool has a disclosure policy.



















