How Are Biologic Drugs Different From "Normal" Drugs?
A biologic drug is a large, complicated molecule that is manufactured within a living organism. That's the easy definition. That being said, it doesn't give you the whole picture of what a biologic is.
The terms biologic and biologic drugs are used to describe a wide range of products, including the latest cancer therapies, vaccines, even whole blood. In fact, it's easier to say what biologics aren't in order to understand them.
Non-biologic, conventional drugs, increasingly called "small-molecule drugs," have an easily identifiable structure, that can be replicated with 100% confidence in scores of manufacturing sites, across the globe.
Another easy way to tell your dealing with a biologic is its route of delivery. If you're supposed to swallow it, it's probably not a biologic.
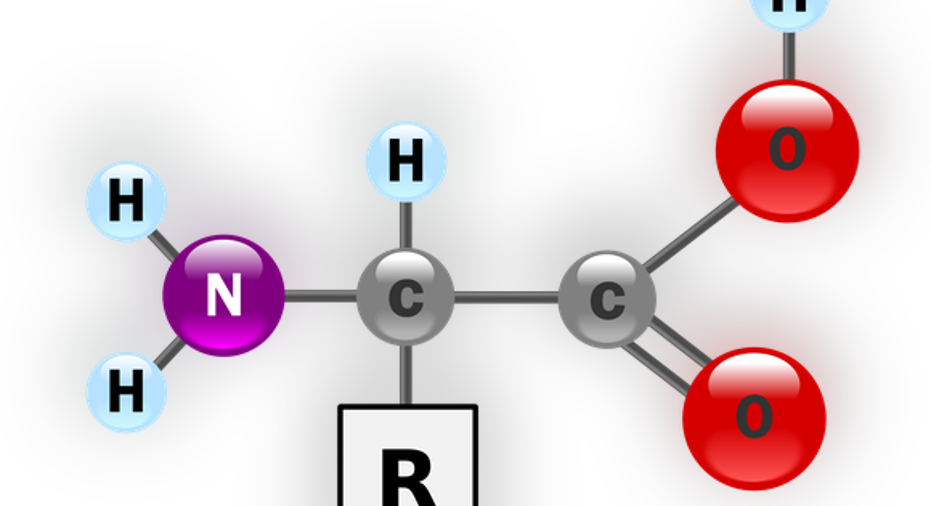
Basic amino acid structure image source: Wikimedia Commons.
With a few exceptions, biologics are so large and complex that their exact structure is unknown, and impossible to recreate with 100% accuracy.
For example, the best selling drug at the moment, AbbVie's Humira, is a human protein that consists of 1,330 amino acids. Even if you know the sequence, we're not playing with Legos.
While the basic structure of a single amino acid is relatively simple (Note the image. Only the "R" part makes them different) when you put a bunch of them together, they start folding into different shapes with what seems like a mind of their own.
Tiny changes in temperature, acidity, and a plethora of unexpected factors can alter the resulting products shape, and its function in the body.This is why these large complex molecules can only be made from living sources, under specific conditions.
Biologic manufacturing processes often involve dozens, if not hundreds of operating procedure controls to create specific conditions. Inserting a specific sequence of DNA into a cloned set of cells, under those conditions, produces the same large, complex molecules every time.This makes the manufacturing process as much a part of a biologic drug, as the drug itself.
Since drugmakers can only manufacture biologics inside another living source, and a specific one at that, they aren't as exposed to competition once their patents expire. Competitors may know the exact sequence of DNA, and the type of microorganism,or animal cell to insert it into, but there is no way to be certain the end product is 100% identical to the biological products they would like to make generic versions of.
This is why the industry insists on the term biosimilar and not generic. It's also the reason regulators require expensive clinical trials before approving one.
This article is part of The Motley Fool's Knowledge Center, which was created based on the collected wisdom of a fantastic community of investors. We'd love to hear your questions, thoughts, and opinions on the Knowledge Center in general or this page in particular. Your input will help us help the world invest, better! Email us at knowledgecenter@fool.com. Thanks -- and Fool on!
The article How Are Biologic Drugs Different From "Normal" Drugs? originally appeared on Fool.com.
the_motley_fool has no position in any stocks mentioned.The Motley Fool has no position in any of the stocks mentioned. Try any of our Foolish newsletter services free for 30 days. We Fools may not all hold the same opinions, but we all believe that considering a diverse range of insights makes us better investors. The Motley Fool has a disclosure policy.
Copyright 1995 - 2016 The Motley Fool, LLC. All rights reserved. The Motley Fool has a disclosure policy.



















