FDA clears Gilead's emergency coronavirus treatment remdesivir
Trials have shown 'positive data' for COVID-19 treatment
Get all the latest news on coronavirus and more delivered daily to your inbox. Sign up here.
An experimental drug, remdesivir, developed by Gilead Sciences and used to treat the Ebola virus was approved Friday for emergency COVID-19 treatment by the Food and Drug Administration.
The emergency authorization allows health care providers to treat patients with “severe” cases of COVID-19, meaning they have low blood oxygen levels or need support like a ventilator.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| GILD | GILEAD SCIENCES INC. | 151.68 | -0.82 | -0.54% |
GILEAD SEES ‘POSITIVE’ DATA ON CORONAVIRUS TREATMENT REMDESIVIR TRIAL
A recent trial found that patients given remdesivir had a lower mortality rate than patients given a placebo – 8 percent compared to 11.6 percent – and recovered 31 percent faster, according to the National Institute of Allergy and Infectious Diseases.
“From day one, the FDA has been committed to expediting the development and availability of potential COVID-19 treatments,” FDA Commissioner Dr. Stephen Hahn said at the White House. “Today’s action is an important step in our efforts to collaborate with innovators and researchers to provide sick patients timely access to new therapies where appropriate, while at the same time supporting research to further evaluate whether they are safe and effective.”
Gilead said it plans to donate 1.5 million doses of remdesivir, which could potentially treat more than 140,000 people. The company is working to rapidly scale up production and supply of remdesivir. It aims to produce at least 500,000 10-day treatment courses by October and 1 million by December, with millions more in 2021 if needed.
Under the terms of the emergency authorization, the drug is suggested for either a five-day or 10-day treatment, depending on the severity of the case.
The U.S. government will coordinate the donation and distribution of the drug to hospitals in the cities hit hardest by the pandemic, according to Gilead.
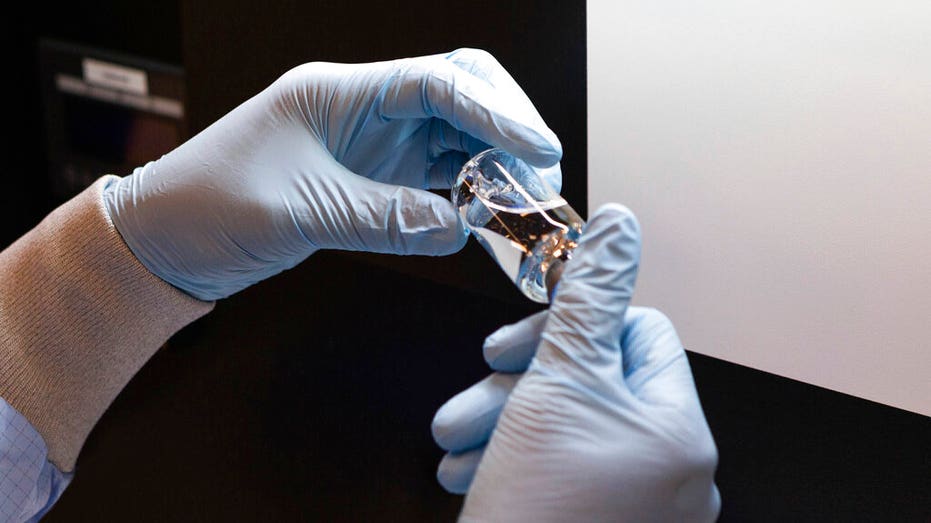
A vial of the investigational drug remdesivir is visually inspected at a Gilead manufacturing site in the United States.(Gilead Sciences via AP)
GET FOX BUSINESS ON THE GO BY CLICKING HERE
“We will continue to work with partners across the globe to increase our supply of remdesivir while advancing our ongoing clinical trials to supplement our understanding of the drug’s profile,” Gilead Chairman and CEO Daniel O’Day said in a press release. “We are working to meet the needs of patients, their families and healthcare workers around the world with the greatest sense of urgency and responsibility.”
President Trump praised Gilead for its contribution in helping people ill with the coronavirus.
“You’re doing great work,” he said. “We’re proud of you.”
Dr. Deborah Birx, an infectious diseases expert with the White House coronavirus task force, said the emergency authorization illustrates what can be done in a short time when needed. The FDA has been issuing similar emergency use authorizations allowing a number of medical products like ventilators to be used to help treat coronavirus patients without typical approval procedures.

Given through an IV, the medication is designed to interfere with an enzyme that reproduces viral genetic material. (Gilead Sciences via AP)
Remdesivir is an antiviral medicine originally designed to treat Ebola. The FDA warned that it can have possible side effects, including increased liver enzymes which may be a sign of inflammation or damage to liver cells, as well as low blood pressure, nausea, vomiting, sweating and shivering.
This post includes previous reporting from FOX Business.




















