Lab recalls over-the-counter heartburn medicine after FDA warns on carcinogen
Pharmaceutical company Dr. Reddy's Laboratories is voluntarily recalling all its over-the-counter heartburn medication in the U.S. due to concerns over potential cancer risks.
The drugmaker said Wednesday that it had been engaged in this recall since Oct. 1. It affects both prescription and over-the-counter ranitidine medication.
The Food and Drug Administration warned in September that some ranitidine medicine had been found to have NDMA, a carcinogen.
Dr. Reddy's makes a generic version of Zantac.
Stocks In This Article:
Retailers like Walmart, Rite Aid and Walgreens have stopped selling the product -- in both forms.
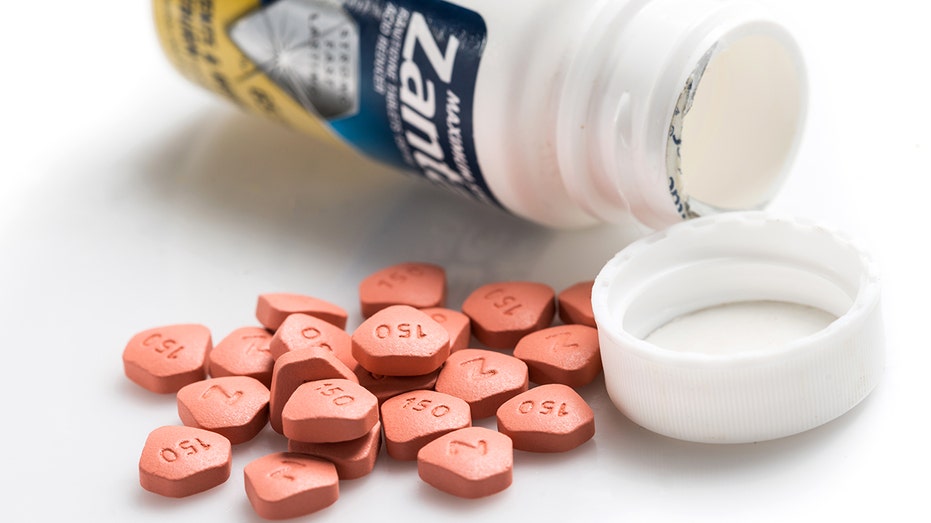
Open Jar of acid reducer medicine Zantac 150 Maximum Strength (ranitidine) on white background. These pills are sold over the counter in USA. Made to treat symptoms of gastro esophageal reflux disease (GERD), persistent heartburn, and other condition (iStock)
Dr. Reddy's said it has not yet been informed of any "adverse events" from customers due to ranitidine and NDMA.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
According to the FDA, NDMA is "classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables."




















