Pharma execs deny cutting corners in vaccine development, praise FDA for not lowering standards
Top AstraZeneca official has seen 'no evidence' FDA is lowering standards
Pharmaceutical company executives denied cutting corners in the coronavirus vaccine development process during a Tuesday briefing before the House Energy and Commerce Committee.
Executives representing pharmaceutical companies working on COVID-19 vaccines, including AstraZeneca, Merck, Moderna, Pfizer and Johson & Johnson, were responding to a question from Rep. Frank Pallone, D-N.J., who suggested President Trump may force the Food and Drug Administration to lower development standards in order to approve a vaccine as quickly as possible.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| AZN | ASTRAZENECA PLC | 193.10 | +5.91 | +3.16% |
| MRNA | MODERNA INC. | 41.01 | +0.14 | +0.34% |
| MRK | MERCK & CO. INC. | 121.93 | +2.18 | +1.82% |
| JNJ | JOHNSON & JOHNSON | 240.00 | +2.26 | +0.95% |
| PFE | PFIZER INC. | 27.22 | +0.73 | +2.76% |
"Historically, I've been very confident in the FDA, but now that Trump is president, I still think there's a real possibility that he will pressure the FDA to lower its standards," Pallone said.
He went on to ask Dr. Mene Pangalos, AstraZeneca's executive vice president of BioPharmaceuticals R&D, what the company would do if the FDA lowered vaccine development standards and approved a drug that is only "10 to 20-percent effective."
FDA TO ISSUE GUIDANCE ON COVID-19 VACCINE APPROVAL
"First of all, all of our interactions with the regulators have given us no evidence that they're lowering the standards or thinking about lowering their standards," Pangalos. "Secondly, as a company, we will always think about safety and efficacy first and foremost and making sure that we have an effective medicine."
Pangalos added that all of the company's studies regarding the vaccine's effectiveness and any changes in FDA requirements would be made public.
WHICH COUNTRY WILL DEVELOP CORONAVIRUS VACCINE FIRST?
Dr. Macaya Douoguih, Johnson & Johnson's head of Clinical Development and Medical Affairs for Janssen Vaccines, similarly told Pallone that COVID-19 vaccine development can be done safely, and the company will monitor the safety of any approved medication in the long-term.
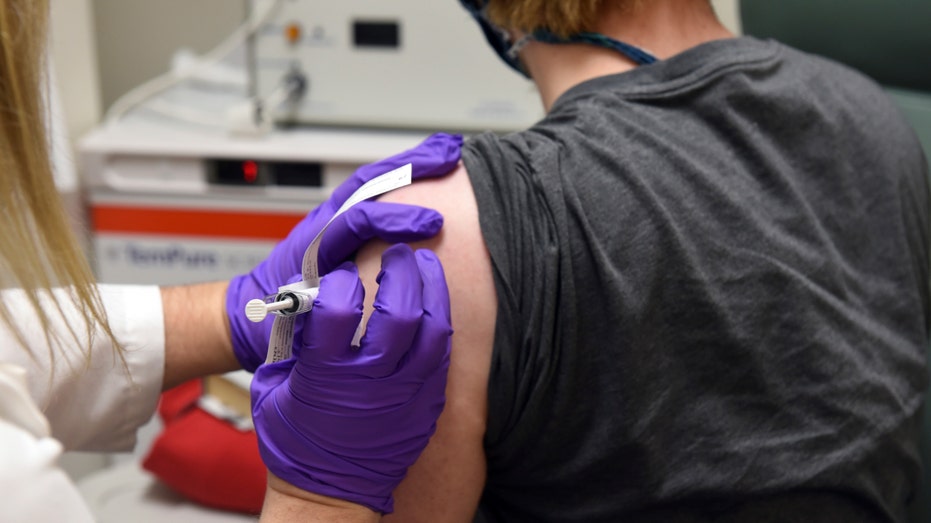
The first patient enrolled in Pfizer's COVID-19 coronavirus vaccine clinical trial at the University of Maryland School of Medicine in Baltimore, receives an injection. (University of Maryland School of Medicine via AP)
"If we saw 10 percent [effectiveness] ... the study would fail if it hit 10 percent," Douguih said. "We would make those results available, but we would not feel comfortable bringing forward a product that was not found to be efficacious according to what we put forth in our protocol.
Pfizer CBO Dr. John Young praised the FDA for its work, saying the administration "should be commended" for its development standards, and that Pfizer has "great confidence in following the FDA guidelines."
US TO ACCUSE CHINA OF ATTEMPTS TO HACK CORONAVIRUS RESEARCH
AstraZeneca is cautiously optimistic that it will have a vaccine by October; Moderna and Pfizer expect to have a vaccine by the end of 2020; Johnson & Johnson expects to have one by March 2021; and Merck shared a general estimate for sometime later in 2021.
As of Tuesday, there have been 3.9 million confirmed COVID-19 cases and more than 143,000 deaths related to the virus. An effective vaccine will likely determine the fate of businesses and schools that have closed due to local lockdowns.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
The Trump administration announced about $10 billion in funding for medical research efforts, including vaccine development, through the Coronavirus Aid, Relief, and Economic Security (CARES) Act in March as part of its "Operation Warp Speed" plan to stifle the pandemic.
Pharma company Novavax announced on July 7 that it would receive $1.6 billion through Operation Warp Speed to develop a vaccine.