Coronavirus vaccine could mean $15B 'windfall' for Pfizer: Report
Pfizer jointly developing drug with BioNTech
Pfizer’s coronavirus vaccine development could result in a big payoff for the drugmaker.
The U.S. government has already put in an order for 100 million doses of an experimental COVID-19 vaccine being developed by Pfizer and BioNTech for $1.95 billion, with an option for 500 million more doses.
| Ticker | Security | Last | Change | Change % |
|---|---|---|---|---|
| PFE | PFIZER INC. | 27.22 | +0.73 | +2.76% |
| BNTX | BIONTECH SE | 106.62 | +1.08 | +1.02% |
PFIZER SALES FALL AS DRUGMAKER RACES TOWARD COVID-19 VACCINE
The vaccine is still pending FDA approval, but officials have given the review process “fast track” designation.
Based off the pricing precedent of just under $20, analysts with Bloomberg Intelligence calculated “a windfall of more than $15 billion revenue for Pfizer.”
“We believe this sets the top price for a vaccine, with lower prices elsewhere,” Sam Fazeli, a lead analyst for Bloomberg Intelligence, wrote in a note. “Need for repeated use would be the game-changer.”
Pfizer has said it’s testing a two-dose regimen for the experimental vaccine.

In this file photo, the Pfizer logo is displayed at world headquarters in New York. (AP Photo/Mark Lennihan, File)
US SECURES 100 MILLION DOSES OF PFIZER, BIONTECH EXPERIMENTAL VACCINE
However, the nearly-$20 price will likely come down over time, assuming there are several equally effective options. Fazeli said in a Bloomberg podcast last week that potential vaccine prices have been negotiated as low as $4 per dose.
“I think $20 per dose sets a precedent for at-profit organizations,” Fazeli said on the podcast.
The coronavirus has sickened more than 16.5 million people and killed more than 655,000 worldwide, according to Johns Hopkins University. That figure includes more than 148,000 deaths in the U.S., the most of any country.
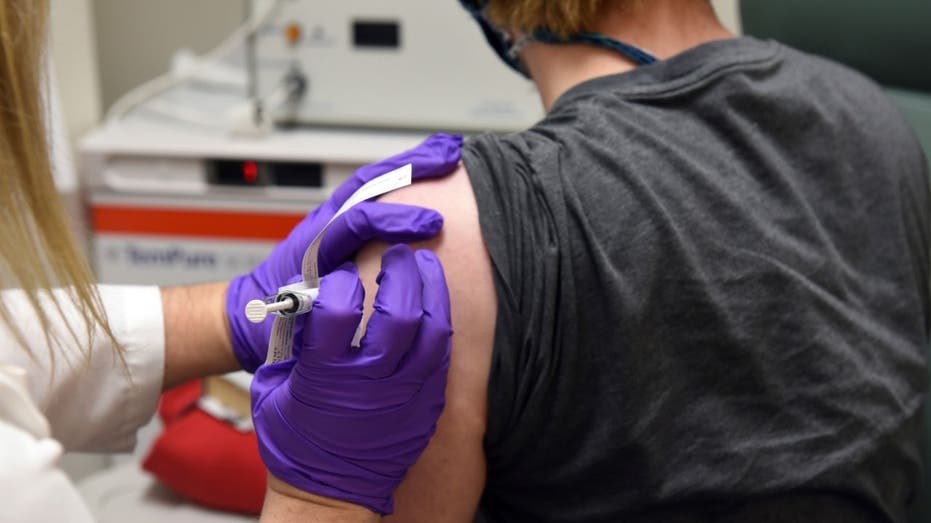
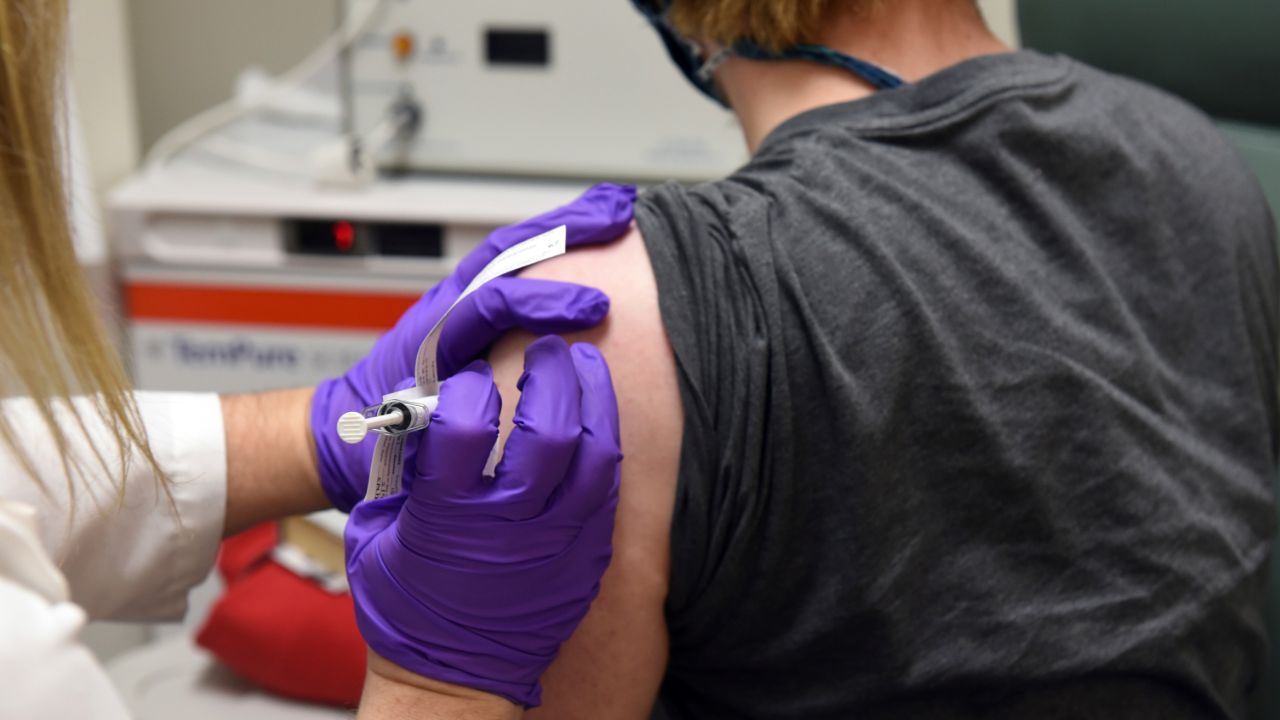
This May 4, 2020 photo from the University of Maryland School of Medicine, the first patient enrolled in Pfizer's COVID-19 coronavirus vaccine clinical trial at the University of Maryland School of Medicine in Baltimore, receives an injection. (Unive
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Pfizer’s experimental vaccine has shown high levels of neutralizing antibody in various species of animals, according to the company. It began Phase 3 testing this week. The global study will include as many as 30,000 participants.
It’s “a major step forward in our progress toward providing a potential vaccine to help fight the ongoing COVID-19 pandemic, and we look forward to generating additional data as the program progresses,” Kathrin U. Jansen, senior vice president and head of vaccine research and development at Pfizer, said in a statement Monday.