FDA approves coronavirus saliva test
First FDA-approved testing method that does not use invasive cotton swabs to collect samples
Rutgers University has developed a novel coronavirus saliva test that will be available at a number of New Jersey testing sites starting Tuesday.
The Food and Drug Administration granted emergency use authorization (EUA) to Rutgers' saliva testing approach, which is the first FDA-approved testing method that does not use invasive cotton swabs to collect samples, the university announced in a Monday press release.
"The impact of this approval is significant," Professor Andrew Brooks, chief operating officer and director of technology development at Rutgers’ RUCDR Infinite Biologics, said in a statement. "It means we no longer have to put health care professionals at risk for infection by performing nasopharyngeal or oropharyngeal collections."
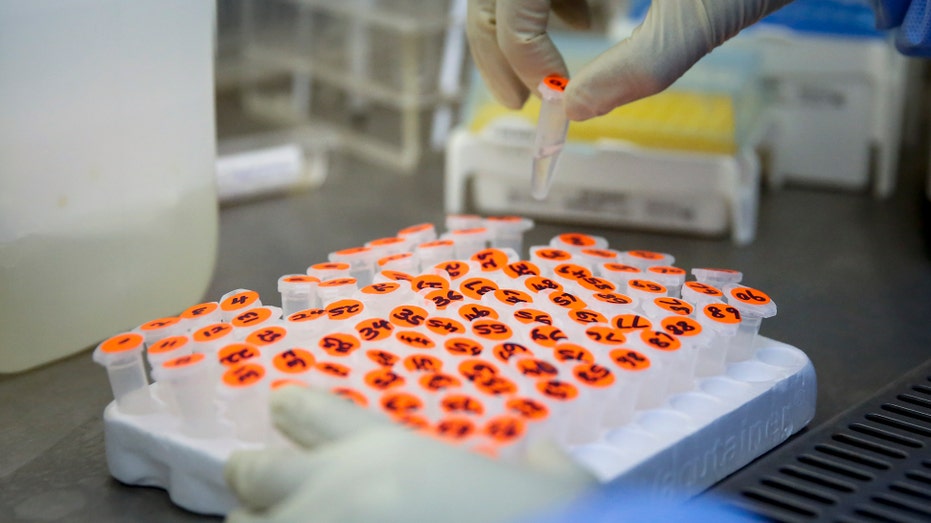
A laboratory technician handles micro centrifuge tubes containing patient samples to be tested for the new coronavirus that causes COVID-19, at the Pathologists Lancet Kenya laboratory in Nairobi, Kenya. (AP Photo/Brian Inganga)
He added that the new method will "preserve precious personal protective equipment (PPE) for use in patient care instead of testing" and "significantly increase the number of people tested each and every day" because patients will be able to self-collect saliva.
WHY IS THERE STILL A CORONAVIRUS TEST KIT SHORTAGE IN THE US?
"All of this combined will have a tremendous impact on testing in New Jersey and across the United States," Brooks said.
The White House COVID-19 task force called Brooks on Saturday after the FDA approved his department's test method to congratulate him and to ask about any specific hurdles his department had to overcome to expand testing in the community.
SOUTH DAKOTA TO TEST CORONAVIRUS TREATMENT IN FIRST STATEWIDE CLINICAL TRIAL
"Shortly" after the call Brooks' office was contacted by some of the world's leading science companies involved in COVID-19 testing, according to the release.

A worker verifies a coronavirus drive-thru testing appointment at a Vehicle Emissions Inspection Program station in Curtis Bay, Md., April 1. (AP Photo/Susan Walsh)
"I have spoken with these companies’ leadership to not only share knowledge but to create opportunities for continuing to help innovate during this crisis," Brooks said. "We will work closely with these new partners, the FDA and the White House task force to leverage everything Rutgers has to offer to not only help our community but also make a global impact."
GET FOX BUSINESS ON THE GO BY CLICKING HERE
The school's new saliva test is expected to help mitigate swab-based testing shortages across the country. It is also expected to present less risk to health care workers since potential COVID-19-positive patients can take the tests at home rather than have one conducted by a medical professional in-person.
"Saliva testing will also be important for people who are in quarantine because they don’t know how long it will be until they are no longer infectious. This will allow health care workers to release themselves from quarantine and safely come back to work," Brooks said.




















