Why Dynavax Technologies Shares Slid 12% in June
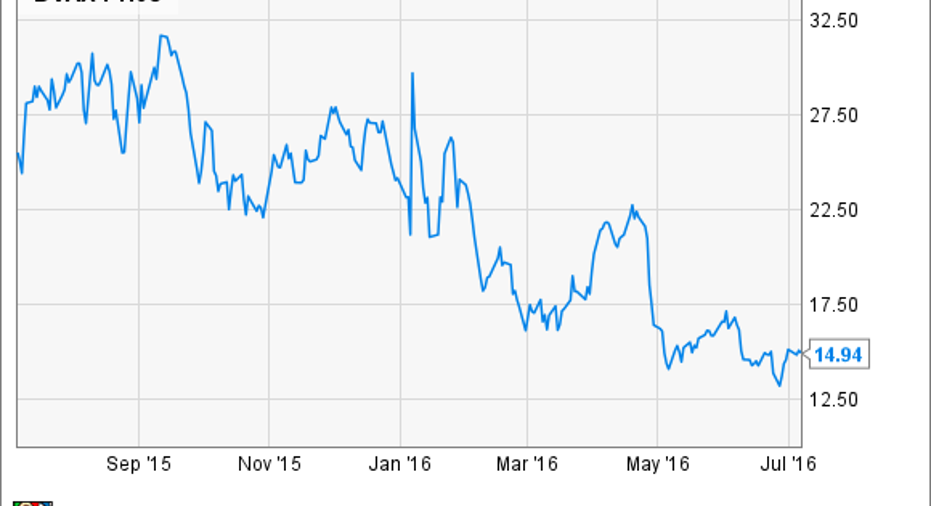
Image source: Stockmonkeys.com via Flickr.
What:After the Food and Drug Administration pushed back its decision date for the company's hepatitis B vaccine in April, shares inDynavax Technologies Corp. have continued to tumble, losing 12% of their value last month, according to S&P Global Market Intelligence.
So what:Dynavax Technologies' investors are eagerly awaiting a go/no-go from regulators on HEPLISAV-B, a hepatitis B vaccine that -- if approved -- could become standard of care.
Currently, GlaxoSmithKline's Engerix-B is widely used to help curb the spread of this highly transmittable disease.An estimated 240 million people have contracted hepatitis Bworldwide, and as a result, hepatitis accounts for roughly 80% of all primary liver cancers.
In spite of the availability of Energix-B, up to 20,000 people in the U.S. will still get infected with hepatitis B. One reason why is Energix-B's dosing schedule places a high burden on patients. Energix-B is given in three doses over six months.
Dynavax Technologies thinks that HEPLISAV-B's dosing schedule would significantly improve patient compliance. HEPLISAV-B is given via two does in one month. HEPLISAV-B may also work better than Energix-B in preventing the spread of hepatitis B. In trials,95% of people receiving HEPLISAV-B were seroprotected, versus 81% of patients receiving Engerix-B.
Despite its advantages, the FDA rejected Dynavax Technologies previous application for approval of HEPLISAV-B in 2013 because of concerns that its use could result in autoimmune disorders.
Given the FDA's prior rejection, it's not surprising that it is approaching HEPLISAV-B's current application cautiously. Earlier this year, it requested additional information from Dynavax Technologies regarding HEPLISAV-B's safety. Unfortunately, when the company supplied that information, the FDA determined it constituted a major amendment to HEPLISAV-B's application. As a result, regulators pushed back the timeline for a decision to December from September.
Now what:The market for hepatitis B vaccines is measured in the hundreds of millions of dollars per year, so an approval could move the needle for this small-cap company. However, a rejection could be a big blow to Dynavax Technologies, and that makes the upcoming FDA decision a binary event.
Since it's unclear whether HEPLISAV-B's advantages can trump any of the agency's lingering safety concerns, buying Dynavax Technologies' stock is a risky proposition. It may be best to join others in sitting on the sidelines on this one until we have a better understanding of the FDA's position on HEPLISAV-B.
The article Why Dynavax Technologies Shares Slid 12% in June originally appeared on Fool.com.
Todd Campbell has no position in any stocks mentioned.Todd owns E.B. Capital Markets, LLC. E.B. Capital's clients may have positions in the companies mentioned. Like this article? Follow him onTwitter where he goes by the handle@ebcapital to see more articles like this.The Motley Fool has no position in any of the stocks mentioned. Try any of our Foolish newsletter services free for 30 days. We Fools may not all hold the same opinions, but we all believe that considering a diverse range of insights makes us better investors. The Motley Fool has a disclosure policy.
Copyright 1995 - 2016 The Motley Fool, LLC. All rights reserved. The Motley Fool has a disclosure policy.



















