The Big News That's Creating Excitement in This Tiny Stock
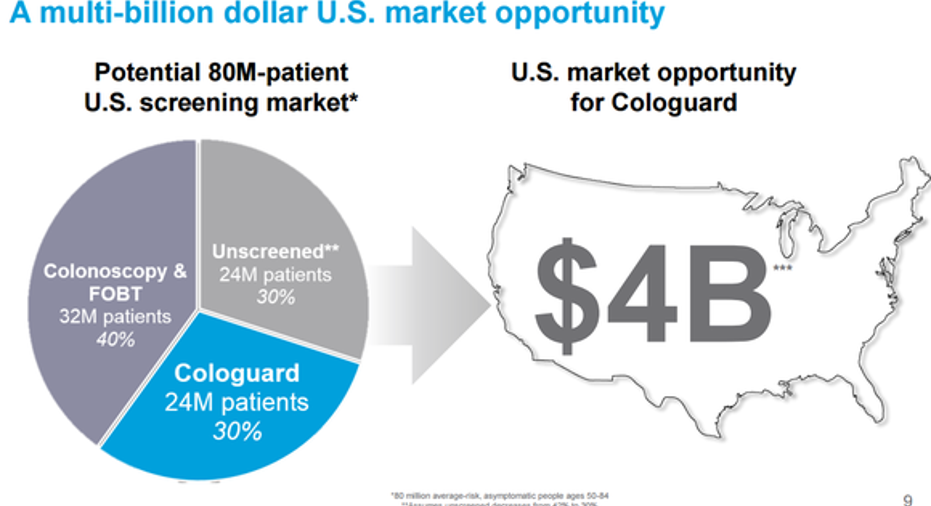
Image source: Exact Sciences.
Exact Sciences investors took a drubbing last year, but new guidance on colorectal cancer screening have ignited a massive rally in the company's shares this month that investors shouldn't ignore. Can Exact Sciences' momentum continue to reward investors with market-beating returns?
The background
Exact Sciences is a diagnostic test maker that markets Cologuard, a colon cancer screening test.The test determines whether or not there's a greater likelihood of colon cancer that would require a colonoscopy. The stool sample used to evaluate the risk of colon cancer is packaged by patients in the comfort of their own homes -- and then mailed to Exact Sciences for testing.
Last year, Exact Sciences completed 104,000 Cologuard tests. This year, the company's management expects that number to climb to 240,000.
Image source: Getty Images.
Changing outcomes
Colon cancer can be easy to treat if it's caught in the early stages. However, many people avoid colon cancer screening. As a result, most cases of colon cancer are diagnosed when patients are older and the cancer has advanced to later stages when it's tougher to beat.By making colon cancer screening more convenient and less intrusive, Cologuard hopes to significantly expand colorectal cancer screening among patients age 50 and up.
Prior to the launch of Cologuard, colorectal cancer screening was done using a fecal occult blood test (FOBT), sigmoidoscopy, barium enema, or colonoscopy.FOBT tests and fecal immunochemical tests (FIT), a more sophisticated variation of FOBT, involve testing for blood in a stool sample; however, this form of testing may not be as good at finding colon cancer as other methods. For example, in trials, Cologuard detected 92% of colorectal cancers and 42% of advanced adenomas, while FIT detected 74% of cancers and 24% of advanced adenomas.
Sigmoidoscopyinvolves inserting a thin tube-like instrument through the rectum into the colon. A barium enemia involves inserting barium into the rectum, and then conducting x-rays; and during a colonoscopy -- the gold standard of screening -- a colonoscope is inserted into the rectum and into the colon. These screening options are more intrusive and expensive than stool testing.
Because of the perceived drawbacks of these other approaches, the percentage of people who should be screened for colon cancer who actually follow through is lower than it needs to be. Only about 60% of patients eligible for screening do it, and that's a big problem given that colorectal canceris the second-leading cause of cancer death in the United States. If more patients embraced screening, then many more lives could be saved.
Delivering the goods
Exact Sciences estimates that the addressable market opportunity for Cologuard is $4 billion; however, payers have been slow to approve reimbursement for the test. As a result, Cologuard's share of that market is undeniably small.Last year, the company conducted 104,000 tests that generated $39.4 million in sales. This year, management thinks it will complete 240,000 tests, and that sales will eclipse $90 million as more insurers sign on.
Image source: Exact Sciences.
The likelihood of capturing a more-meaningful share of that market opportunity may have just improved following an update to theU.S. Preventative Services Task Force (USPSTF) colorectal cancer-screening guidelines.Last year, preliminary guidelines on colorectal cancer screening from the USPSTF labeled Cologuard an alternative screening option to other screening choices, such as FBOT and FIT. That preliminary guidance could have crimped Cologuard's market potential if it had been finalized.
Fortunately for Exact Sciences investors, management appears to have successfully lobbied the task force to drop the words "recommended" and "alternative" from its final guidelines, which were reported this week. By elevating Cologuard to the same level as prior-generation screening methods, a roadblock that insurers could have used to restrict coverage has been removed, and the likelihood that Cologuard gets relegated to a niche test used in patients who refuse undergoing the more-invasive screening options is reduced.
Looking forward
Cologuard can be better at finding colorectal cancer than standard fecal testing, and it's more patient friendly than the intrusive screening methods. That's potentially a big win for patients. If those advantages lead to more people getting screened, the likelihood of catching colon cancer early jumps.
It's anyone's guess if this finalized guidance will cause Cologuard prescriptions to surge, but the news is bullish for investors because less-favorable guidelines could have limited Cologuard's peak sales. Before investors addExact Sciences to portfolios, however, they're going to want to take a close look at the company's income statement.
Exact Sciences is spending money at a rate that's north of $200 million per year, and that's more than twice what management hopes to haul in from Cologuard in sales this year. Because losses are mounting, a good plan may be to wait and see if test demand accelerates now that the guidelines have been released. If they do, then it could shorten the runway to profitability for this company.
The article The Big News That's Creating Excitement in This Tiny Stock originally appeared on Fool.com.
Todd Campbell has no position in any stocks mentioned.Todd owns E.B. Capital Markets, LLC. E.B. Capital's clients may have positions in the companies mentioned. Like this article? Follow him onTwitter where he goes by the handle@ebcapital to see more articles like this.The Motley Fool has no position in any of the stocks mentioned. Try any of our Foolish newsletter services free for 30 days. We Fools may not all hold the same opinions, but we all believe that considering a diverse range of insights makes us better investors. The Motley Fool has a disclosure policy.
Copyright 1995 - 2016 The Motley Fool, LLC. All rights reserved. The Motley Fool has a disclosure policy.



















