Teenagers are latest participants in COVID-19 vaccine trials; safety concerns arise
Cincinnati Children's Hospital has seen promising results among children receiving Pfizer experimental vaccines
COVID-19 vaccine trials have shown promising results on adults thus far, but testing on children is just getting started.
Clinical testing on children is the next major step among top candidates like Pfizer and Moderna, which have proven to meet the safety and immune response thresholds. After the Pfizer vaccine appeared to be nearly 95% effective in adults, the Cincinnati Children’s Hospital became one of the few sites to begin testing on adolescents.
Stocks In This Article:
'GAME-CHANGER' CORONAVIRUS VACCINE COULD BE SOON ON THE WAY
Since April, the clinic has been one of five Pfizer pediatric testing sites working on COVID-19 vaccine trials on teenagers. A month into the clinical trial has already produced favorable results, signaling a nod to expand to even younger age groups.
“What we've seen so far is that safety has been very similar to the adults,” Dr. Robert Frenck told FOX Business. “We are seeing that some people are having aches and pains but nothing has been serious or caused a child to miss a day of school. We are very excited about that, and it really gives us heart that we will be able to move down further in age and actually may even need to go down as young as infants eventually to try to really stop this whole outbreak.”
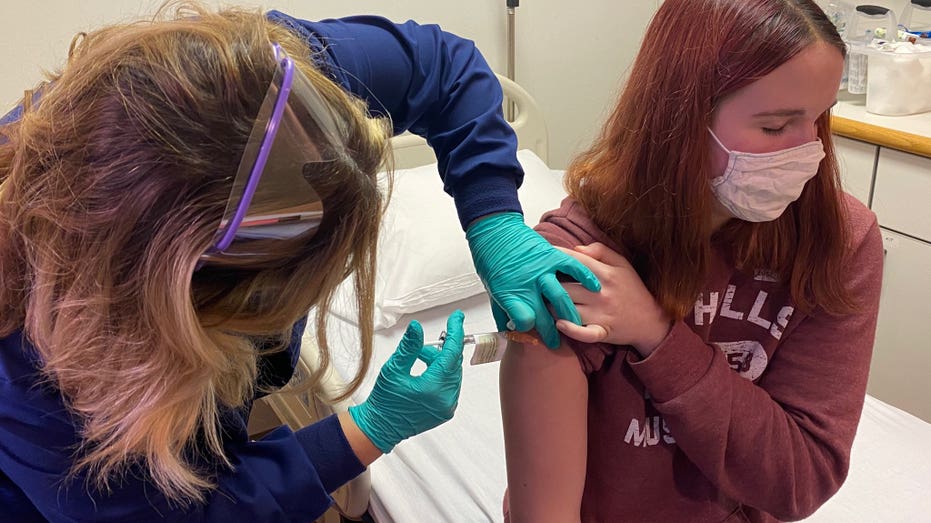
A 16-year-old patient receives a dose of Pfizer's coronavirus vaccine candidate at the Cincinnati Children's Hospital. (Photo courtesy of Cincinnati Children’s)
US FDA GRANTS EMERGENCY USE AUTHORIZATION TO REGENERON COVID-19 ANTIBODY GIVEN TO TRUMP
Around 400 children, ages 16 and 17, have enrolled in the study, which administers two doses of Pfizer’s experimental vaccine, in addition to about 100 children in the 12 to 15 age group. After the safety review confirms that testing can continue, the clinic will aim to enroll up to 2,600 between the ages of 12 to 18 nationwide.
Even though only about 13% of all participants have experienced mild side effects, with 87% showing no side effects at all, safety has become a critical focus to parents who still hold concerns about a non-certified vaccine. Among some of the setbacks include the potential side effects and whether or not the vaccine will give children COVID-19.
“We absolutely say this vaccine cannot give you COVID-19 because it's not a live virus,” Dr. Frenck said. “What every parent asks and what I'd expect them to ask is what's the side effects? And so we tell them what we know, what the adults have seen so far and what the children should expect.
COVID-19 SHOTS COULD REACH US BY MID-DEC
So far, the safety has been identical to testing on adults, and Dr. Frenck anticipates that both children and adults will receive the same doses of vaccine.
According to Dr. Frenck, the clinic is encouraged by the line of future enrollees it has seen, even as some parents express reluctance and prefer to wait for their children to receive any form of vaccination.
On the other hand, many have raised questions of the need to test children in the first place since most are not getting sick. Just over a million children have documented infections in the United States, or 11.5% of all COVID-19 cases, with over 9,000 children hospitalized and nearly 150 fatalities.
“We need to test children and to vaccinate children because while it might not be a high rate, children are getting infected, children are getting sick and children are dying,” Dr. Frenck said. “We need a vaccine to not only protect the children directly, but also to protect the children from spreading to their family unknowingly.”
In order to get children back to school, the parents and teachers also need to feel they are safe, and a vaccine is the way to make people not only feel safe, but keep the virus at bay, Dr. Frenck said.
CLICK HERE TO READ MORE ON FOX BUSINESS




















