COVID-19 rapid test recalled for 'high number' of false positive reports
FDA said the COVID-19 at-home test recall was due to a 'high number' of false positives
FOX Business Flash top headlines for March 18
Here are your FOX Business Flash top headlines for March 18.
The Food and Drug Administration announced Wednesday that a healthcare company has recalled 45,500 COVID-19 rapid tests due to a "high number of false positive reports."
Pharmaceutical company Celltrion USA announced on Feb. 28 it is recalling specific lots of the DiaTrust COVID-19 Ag Rapid Test due to the high number of false-positive reports, an FDA recall webpage read on Wednesday.
The FDA says that a false-positive test result can lead to a delay in "the correct diagnosis and treatment for the actual cause of a person's illness."
MODERNA SEEKS FDA AUTHORIZATION FOR FOURTH COVID-19 SHOT

Home test kits, specifically Celltrion DiaTrust COVID-19 Ag home tests, stacked for delivery in Boston on Jan. 5, 2022. The Neighborhood Villages organization is distributing around 40,000 home COVID-19 test kits provided by the state to child care (Photo by Lane Turner/The Boston Globe via Getty Images / Getty Images)
The COVID-19 rapid tests also displayed a shelf life of 18 months, but the FDA's emergency use authorization states that the tests can only be used for 12 months.
Individuals and customers who received the effected COVID-19 rapid tests are being told to discontinue use and return the unused products.
"The use of the affected product could cause serious adverse health consequences and death," the FDA webpage states.
PFIZER-BIONTECH SEEK AUTHORIZATION FOR SECOND COVID-19 BOOSTER

A sign for the Food And Drug Administration is seen outside of the headquarters on July 20, 2020 in White Oak, Maryland. (Photo by Sarah Silbiger/Getty Images / Getty Images)
On Wednesday, the FDA also announced the recall of the SD Biosensor STANDARD Q COVID-19 Ag Home Test, stating the test is not approved by the FDA for marketing or distribution in the United States.
Since the test was not authorized by the FDA, the government agency said "there is not sufficient data demonstrating that the test's performance is accurate," which could lead to inaccurate results.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
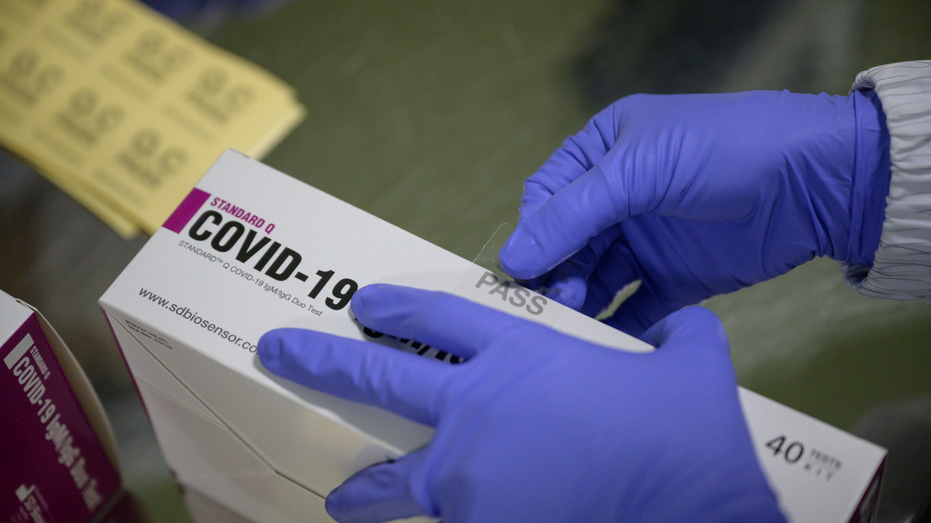
VID-19 novel coronavirus testing kits are packaged on a production line at the SD Biosensor bio-diagnostic company near Cheongju, south of Seoul on March 27, 2020. - SD Biosensor is one of five companies in South Korea -- which appears to have brough ((Photo by ED JONES/AFP via Getty Images)
"This means there is a risk of both false-negative and false-positive test results. False negative results are when the test does not detect the SARS-CoV-2 virus but the person is actually infected. False-positive results occur when the test says the person has SARS-CoV-2 virus present, but they are not infected," the website states.




















