Coronavirus vaccine delivery: The journey from the factory to hospitals, in pictures
Shots made by Pfizer Inc. and its German partner BioNTech are the first authorized for emergency use by the Food and Drug Administration
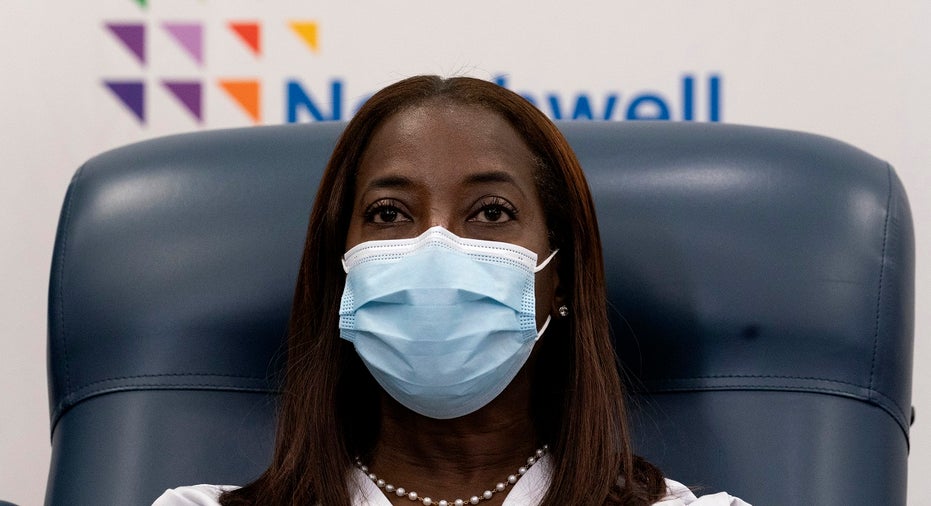
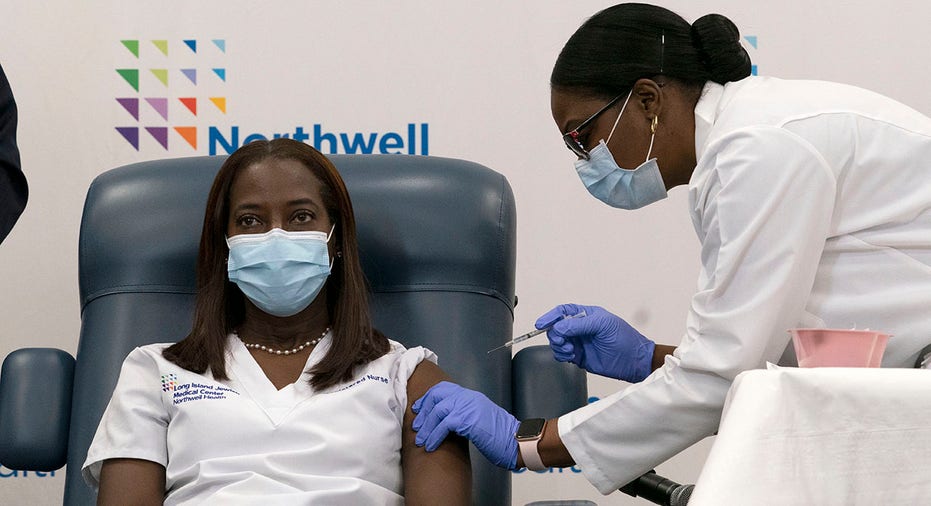
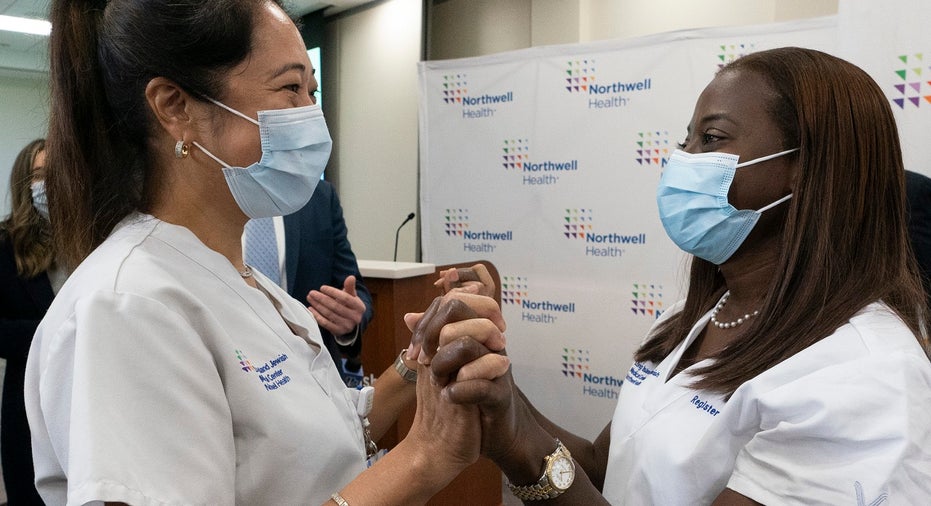
A New York City nurse became the first person in the state to receive Pfizer's COVID-19 vaccine on Monday.
Sandra Lindsay was given the injection at the Long Island Jewish Medical Center in Queens as part of a widespread effort to immunize health care workers and nursing home residents.
Hospital workers begin unloading precious frozen vials of COVID-19 vaccine Monday morning, with the first vaccinations against a scourge that has killed nearly 300,000 Americans expected later in the day.
“It feels like the cavalry is arriving,” Robert C. Garrett, CEO of Hackensack Meridian Health, said as New Jersey’s largest health network awaited delivery.
Shots made by Pfizer Inc. and its German partner BioNTech are the first authorized for emergency use by the Food and Drug Administration -- beginning what will become the largest vaccination campaign in U.S. history. Several other countries also have OK'd the vaccine, including the U.K., which started vaccinating last week.
For health care workers who, along with nursing home residents, will be first in line for vaccination, hope is tempered by grief and the sheer exhaustion of months spent battling a coronavirus that still is surging in the U.S. and around the world.
Here's a look at some of the images of the journey from the manufacturing plant to hospitals.
Factory Farewell
Dry ice is poured into a box containing the Pfizer-BioNTech COVID-19 vaccine as it is prepared to be shipped at the Pfizer Global Supply Kalamazoo manufacturing plant in Portage, Mich., Sunday, Dec. 13, 2020. (AP Photo/Morry Gash, Pool)
Packed in dry ice to stay at ultra-frozen temperatures, the first of nearly 3 million doses being shipped in staggered batches this week made their way by truck and by plane around the country Sunday from Pfizer’s Kalamazoo, Michigan, factory. Once they arrive at distribution centers, each state directs where the doses go next.
Some hospitals across the country spent the weekend tracking their packages, refreshing FedEx and UPS websites for clues.
On the Move
In this late Sunday, Dec. 13, 2020, photo provided by Los Angeles World Airports, a FedEx Airbus A300F4-605R carrying the first batch of COVID-19 vaccine arriving in Los Angeles, is seen at Los Angeles International Airport. (Los Angeles World Airports via AP)
More of the Pfizer-BioNTech vaccine will arrive each week. And later this week, the FDA will decide whether to green light the world’s second rigorously studied COVID-19 vaccine, made by Moderna Inc.
Now the hurdle is to rapidly get vaccine into the arms of millions, not just doctors and nurses but other at-risk health workers such as janitors and food handlers — and then deliver a second dose three weeks later.
“We’re also in the middle of a surge, and it’s the holidays, and our health care workers have been working at an extraordinary pace,” said Sue Mashni, chief pharmacy officer at Mount Sinai Health System in New York City.
Plus, the shots can cause temporary fever, fatigue and aches as they rev up people's immune systems, forcing hospitals to stagger employee vaccinations.
Delivery Complete
A FedEx driver gives a thumbs up after delivering a box containing the Pfizer-BioNTech COVID-19 vaccine to pharmacists Richard Emery, right, and Karen Nolan as it arrives at Rhode Island Hospital in Providence, R.I, Monday, Dec. 14, 2020. (AP Photo/David Goldman)
The FDA, considered the world’s most strict medical regulator, said the Pfizer-BioNTech vaccine appears safe and strongly protective -- and laid out the data behind it in a daylong public meeting last week for scientists and consumers alike to see.
Still, emergency use means the vaccine was cleared for widespread use before a final study in nearly 44,000 people is complete -- and that research is continuing to try to answer additional questions. While effective against COVID-19 illness, it’s not yet clear if vaccination will stop the symptomless spread that accounts for half of all cases.
Nurse Sandra Lindsay participates in a conference call with Gov. Andrew Cuomo after she was inoculated with the Pfizer-BioNTech COVID-19 vaccine, Monday, Dec. 14, 2020, at the Jewish Medical Center, in the Queens borough of New York. (AP Photo/Mark Lennihan, Pool)
The shots still must be studied in children, and during pregnancy. But the American College of Obstetricians and Gynecologists said late Sunday that vaccination should not be withheld from pregnant women who otherwise would qualify.
While the vaccine was determined to be safe, regulators in the U.K. are investigating several severe allergic reactions. The FDA’s instructions tell providers not to give it to those with a known history of severe allergic reactions to any of its ingredients.
The Associated Press contributed to this report