The Motley Fool Sits Down With the CEO of Dexcom Inc.
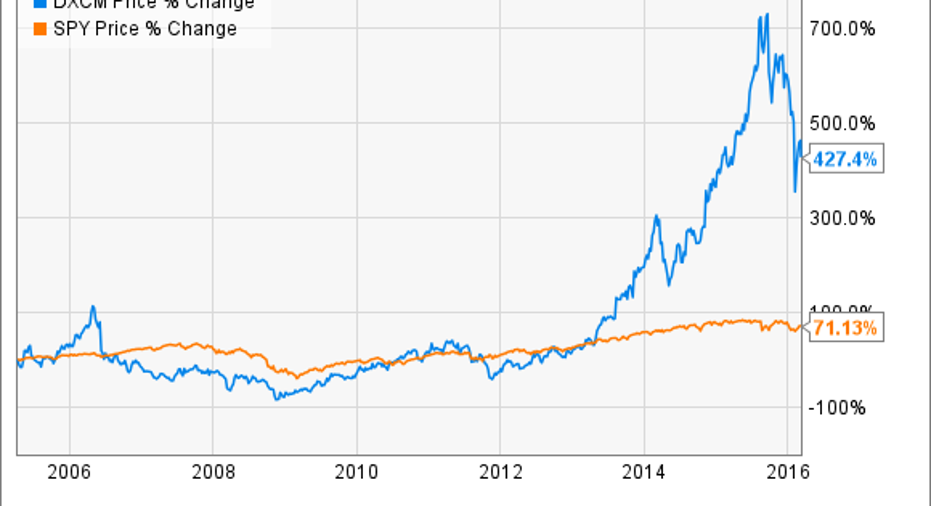
Image source: Dexcom.
The Centers for Diseases Control and Prevention estimates that more than 29 million Americans have diabetes,a chronic disease that inhibits the body's ability to produce or effectively utilize insulin. Patients with diabetes often have difficulty regulating the amount of glucose in their blood, which can lead to a variety of adverse health outcomes.
Treating diabetes is both complicated and costly. The American Diabetes Association estimates that we spent $245 billion to treat diabetes in 2012 alone, which was roughly one out of every five dollars spent on healthcare.
One of the most interesting diabetes-treatment technologies that has emerged in recent years is the continuous glucose monitor, or CGM. These devices are worn on the body and constantly monitor blood glucose levels, which gives patients and providers data that they need to make therapeutic decisions.
Dexcom(NASDAQ: DXCM), a mid-cap company that has been growing like a weed for more than a decade, is a leader in the CGM space. The company has been a home run for investors, up more than fourfold since it came public and smashing the returns of the market in general.
I recently sat down with Kevin Sayer, the CEO of Dexcom, to discuss where his company is today and what kind of innovations are possible in the years ahead.
Following is an edited transcript of our conversation.
Brian Feroldi: If you could just quickly update us on some of your recent results. You said on the call, revenue up 10 times from 2010. You guys were GAAP profitable for the, I think, second time ever --
Kevin Sayer: Second time ever.
Feroldi: -- during the quarter. Congratulations on that. You have, what, 140,000-plus patients, something like that, around the world you estimated, and for 2016, you're forecasting 35% growth?
Sayer: 35% to 40%.
Feroldi: That's phenomenal.
Sayer: A revenue range of $540 million to $565 million.
Feroldi:Obviously, the business is really firing on all cylinders, and personally, I've been incredibly impressed with how fast you've grown. A couple of years ago, I remember, I believe it was Terry [Gregg, Dexcom's previous CEO] who said you guys set an internal target of something like 40% annualized growth for the company. I kind of chuckled to myself, like, "Well, all right."
Sayer: That's still what we do, because if you grow 40% every year, you double every two years. That's how we look at it. Those 40%s, 40% of $400 million is a lot more than 40% of $250 [million] was last year, so those targets get big, but we're still so under-penetrated, even in our U.S. core market, and there's plenty of space for growth there. With the cadence of new products we have coming out combined with the cadence of products we've launched the past couple years, we'll address different patient needs, which will enable us to expand and, we believe, keep that up.
Feroldi: All right. That's great. Your most recent offering is called the G5 with Share?
Sayer: G5 Mobile with Share. In fact, I'm wearing a sensor today. I don't know if you've seen this, but that is what a patient experiences with the G5 app.
The G5 system, for example, that's the glucose value. You can see I don't move much. Here's an interesting thing you'll appreciate. This is the first medical device ever without a printed user guide. It's all right there available at your fingertips on the phone. If you have a problem, you want to call Dexcom, you touch that, you touch that, you hit "call," and you've got them on the phone.
Feroldi: That's nice.
Sayer: This makes the interaction for somebody with diabetes and the device very similar to what they have in the rest of their life, and it makes it much easier. It'll just get better and better and better as we learn.
We're only on iPhone now. We will file the Android app in the first half of this year and hopefully get it approved toward the end of the year. Then, the Follow app, to be able to follow people, is available on Android and on Apple.
Feroldi: That's just through the cloud, correct?
Sayer: That's through the cloud. Again, with this app, the data transmits to the cloud. If I turn it on, that symbol here, the triangle, is the Share app. I'm not sharing my data with anybody right now, but that's a multicolor triangle. Then, you pick up to five people you share your data with. Then, those followers and the app for the people that follow you is called Follow. They set their own alert alarm settings. For example, if you're a parent and you want to follow your kid, rather than setting the alert every time they go below 70, you can say, if they go below 70 for an hour.
What's been interesting about Share is the psychological dynamic we created among families, because parents are always worried about their kids with diabetes, and some parents have been very, "OK, I've got Share. I can know. This is good. It gives me comfort." Others have become micromanagers, and so kids have to make rules. My cousin's daughter has type 1 diabetes, and she's on the Dexcom system, and I got them on the Share system. When I talked to her after she got it, I said, "So, do you let your dad follow you?" "Yeah." Her grandparents, or my aunt and uncle, I said, "How about your grandparents?" She said, "Noooo."
Her mom's a math teacher, and her mom's more numbers-driven than my cousin is. They're divorced. "Do you let your mom follow you?" "Working on that one." It is a different dynamic, and for kids at school, at college in particular, they'll just turn the parents off for the weekend, and then the parents go, "What's going on here?" Married couples, the married couples have to have rules. "You can't bug me. You could do" -- it's a dynamic that we thought might happen but we really weren't prepared for. The anecdotal conversation's really fun, but what a valuable tool.
Feroldi: Sure. Obviously, you still have to calibrate the system. Can you do that right on the app?
Sayer: Twice a day. That feature right there, you just enter your blood glucose value right there as it's showed. I hit the meter. Since I don't have diabetes, I'll just enter what I was going, and there it is. The calibration's factored in. You enter it right there.
Feroldi: OK. That's great.
Sayer: Just so much easier.
Feroldi: That obviously creates a unique option for, say, providers. Do you guys allow providers to follow their patients as well?
Sayer: We allow five followers for every patient, and providers can follow if the patients want them to and invite them to. It raises a very interesting question. Having been around diabetes yourself, there is a very divided feeling among endocrinologists, "Do I every want to see all that data real-time?"
Feroldi: That's my next question.
Sayer: There are a few physicians who follow patients when they start them up on CGM. There are others I've talked to who, they don't want to be that involved in their patients' lives. They more want to teach them how to run their lives rather than get involved. It's a mix across the board.
Feroldi: ... because it could open up some potential legal issues.
Sayer: Liability. Yeah.
Feroldi: Absolutely. Especially for, say, a small endocrinology practice that has one provider versus a big office that has multiple staff and can do it.
Sayer: Absolutely.
Feroldi: Technologically, it's there ...
Sayer: It's open for the patient and the care provider to decide what they want to do.
Feroldi: OK, that's great. That's really exciting. Obviously, it's a big hit. This product was also launched worldwide?
Sayer: It was launched in our five biggest countries in Europe, yes. We launched them both at the same time.
Feroldi: OK. What do payers think of this? You guys have obviously had a lot of success with payers in the U.S. I know that you're still having some problems with international.
Sayer: You know what? Internationally, we've achieved a good level of reimbursement in Sweden, and our business more than doubled in that geography last year, so it has been great where we have reimbursement. We're working hard on Germany, France, and the U.K., and we'd like to see something happen certainly over the next 12 months in a couple of those countries.
The other places, there's some limited reimbursement in the Netherlands. There is reimbursement in Switzerland, but it's country to country, and it's one by one. They may pay for it differently and may put different restrictions around CGM. It's tough to manage. That's why we are expanding and setting up a European headquarters.
Feroldi: Scotland, correct?
Sayer: Yeah, Scotland's going to be our choice of our headquarters. Then, we'll add bodies in some of these other countries because we really need more people on the street to hear what's going on. We can't let people determine the fate of our product without us really being involved. While our distributors have been great, we've grown our international business as fast as our U.S., and we have maybe four or five international employees total. The distributors have done a great job, but we need feet on the street for clinical studies, for reimbursement, for stuff like that so we can be involved in the process and know what's going on.
Feroldi: For educating providers, providing clinical support, all those kind of things that are obviously super-critical to getting the product off the ground.
Sayer: We just need to do a little more. That's why we're going to do that overseas.
Feroldi: How is reimbursement in the U.S. going? I know that you guys enjoyed pretty wide --
Sayer: We're covered by 98% of private pay and about 15 states for Medicaid now.
Feroldi: Fifteen?
Sayer: Fifteen, yeah. Medicare, we're working on, and we can talk more about that later if you'd like. I would say with the payers, the coverage is, by and large, good. It goes in cycles. For some period of time, one payer would we real good, and another one would give you trouble. Anytime you bill insurance directly, that's what you're going to deal with.
We very publicly stated our goal is to move CGM to the pharmacy because we think it'll be much easier for a patient to go to the drugstore, order their CGM supplies, and pick them up there if they're in stock or have them delivered the next day, and have that be the reorder process. If you have any prescriptions at a pharmacy, you don't ever get the opportunity not to reorder. It just happens.
We think that would be a much better reimbursement model for our patients. We've got several of the payers to flip to pharmacy benefit, but it's been slower than we'd like. We've talked about on some of our previous earnings calls there's inconsistent interpretation of pre-authorization policies. For example, one geography will say, "OK, we don't need to do anything, and we'll approve this." Others will apply them strictly. It's really a question of time and training, but over time, that's where CGM needs to be purchased. People need to go be able to just pick it up.
Feroldi: Sure. From a cost perspective, obviously it's a higher direct cost, but one would think that given that CGM does change the patient's behavior, hopefully it prevents the highs, it prevents the lows, and you could eventually show that there's a cost savings in the long term to getting them on CGM?
Sayer: That's a great point. Cost-benefit over time in our industry's going to be extremely important. The drug companies are all facing that now as you read everything going on at all the diabetes drug companies. The cost-benefit equation in the U.S. is relatively simple if you just look at hypoglycemia. If it costs X dollars a year, and let's say a patient spends between $2,500 and $5,000 a year, depending upon what equipment they buy and how long they wear their sensors, but for full-time use, if you avoid one serious hypoglycemic event and one hospitalization, that's $15,000 to $20,000.
We can justify on hypoglycemia spending alone, pretty much cost justify that in the U.S. That's tougher in Europe, where hospitalization doesn't cost near as much money, but over time, the health benefits of staying within a tighter range I think will prove to have an excellent cost-benefit as well.
We've got a study going on in Europe now with the Swedish investigator where he's run patients for a long time on CGM where we'll get some longer-term healthcare benefit data that should be useful for reimbursement in Europe. We've got a study going on in the U.S. that we call the Diamond Study, where we're taking 200 patients, and they're multiple-daily injection patients, putting them on CGM and comparing them to another 200-patient cohort and comparing everything about those patients for a six-month period. We'll publish the first data on that study in the second half of the year, but that'll be a series of publications over a year because there's smaller groups within the study, and it will take a while to finish it all, but we believe we can document the utility of CGM in these studies.
A1C coming down is always a good benefit, and it's nice, but some people run too low. If you run too low and your A1C goes up a point or half a point, but you avoid a bunch of hypo events, that's a better outcome, too. I think we'll see a lot of good data from that study, and we see great data. A lot of drug companies use CGM in their drug trials as a secondary outcome, it's not a primary outcome yet, to watch the glucose variability in the patients that they're treating.
We've seen very fascinating results with a lot of the compounds that are discussed in the investment community, all these new type 2 drugs. You run them a month on CGM without the drug, and then you run them at various times of the trial, and you see what the drugs do. Very good measure and remarkable results. That's one of the reasons we believe there's a great type 2 market for us over time as well.
Feroldi: Sure. To me, this is something that payers should be jumping at. I know that currently, depending on the payer, there's lots of documentation that they demand, which slows down the process, or they push higher and higher copays toward the patient. Is the goal to take a study like this, and if it shows a cost-benefit, to take that to payers and say, "Let's remove these barriers, let's get more people on it"?
Sayer: Yes. We would love to do that with the study. I think the neatest thing, though, about this Gen 5 system and where we're headed with our technology, we're ultimately not going to need the studies. We'll have the data for every patient in the payer's database over time. One of the investments we say we're going to make this year is in an advanced data platform. When we get that advanced data platform built, one of the key legs of that platform is going to be payer data.
Theoretically, we could walk into Cignaand say, "You have 22,312 patients on our system. Here's how they're doing, and here's your 500 problem patients, and boom, b-boom, b-boom." I can even see the day sometime where that'll be part of the equation in getting CGM reimbursed that we have to show those type of outcomes and have that type of interaction with the payers.
Feroldi: That's wonderful, and I think that's, like you said, with going to the pharmacy will also increase convenience, and ...
Sayer: Accountability, because through a pharmacy system, as we have learned at the payers, through the pharmacy information systems, they track every single prescription and everything. It's not quite the same on the durable medical equipment side. For example, they can't tell whether a patient uses a Medtronic (NYSE: MDT) or a Dexcom sensor. They both have the same reimbursement code in the system, whereas in pharmacy, we'll have an individual unique identifier that can track us. We think there's a bunch of other benefits there.
Feroldi: That's great. On your call, you mentioned that obviously you've been working toward Medicare. It's been slow, but you guys are taking a three-pronged approach. Is one of the big barriers there that they want you to have a replacement claim where patients can dose off the CGM data?
Sayer: That is the barrier that CGM established initially that since you can't dose off this, you're not replacing anything else, so it looks like --
Feroldi: It's just an additional cost.
Sayer: -- an additional cost to the system. Our first step on that, and that's the administrative arm of our three-pronged approach, is to try and get a non-injunctive claim. As we said on the earnings call, we've had meetings with the FDA for quite a long time on this matter, and we're going to learn a lot in the next two or three months, and in May, when we have the earnings call, we'll talk a lot more about it and give you guys, everybody, some clearer guidance as to what we think's going to happen.
Feroldi: You already have that claim in Europe. Is that correct?
Sayer: We have that claim in Europe.
Feroldi: OK, so there's a precedent already for --
Sayer: There's a precedent there.
Feroldi: -- a regulatory body giving you the thumbs-up on that?
Sayer: Yeah.
Feroldi: OK. Is that just based on, essentially, accuracy data when compared to finger sticks?
Sayer: It's based on the accuracy data that we have filed in Europe previously. One of the reasons we're comfortable with the dosing claim is we have run... first of all, we have a huge database of all the clinical trials that we've run, so we know under various scenarios what can happen if a patient makes an error of some kind.
For example, you just saw my CGM value, and I calibrated it, the 97 number that was there. What happens if you put 300 in instead of 97, because you had syrup on your finger from the pancake that you ate? We have algorithms; we can then simulate your data and say, "OK, this is what would happen, and this would be the error. What would happen to the patient in the event that their readings were that much too high?" We've simulated data on the low side as well.
There's a lot of science behind our belief that our device is good enough for a dosing claim now. The FDA's indicated to us that the current G5 system with the current algorithm should be accurate enough, and we've had a lot of discussions about the data required to get there and post-market data that they would be interested in. We'll know more about that. Like I said, there's meetings scheduled over the next two months that are going to be critical in that process. In May, we'll say more.
A lot of our patients dose now, and surveys that I've seen done by independent entities outside of us ask the question, "Do you dose with your CGM in there?" There's a lot of "yeses."
Feroldi: That means they're being honest.
Sayer: That means they're being honest. FDA knows this. That's why they're pushing us to get the claim and determine the limits of the system that, is it really good enough to do that? Those are the things we're working on with the agency.
Feroldi: OK, that's great. Do you ultimately see you guys making a push into the hospital market, if somebody came into the hospital with diabetes, they'd stick one of these on them?
Sayer: Yeah. In fact, when Terry came back to the business in 2007, that's what he wanted to do. He really felt this could grow rapidly as a hospital-based technology, and it didn't turn out that way. We had a partnership with Edwards Lifesciences that has gone by the wayside, but I believe with our new technologies, the Gen 6 sensor is a platform where the algorithm can be adjusted to have no calibrations, for example. We've simulated data. We've run studies and looked at it. You might be able to just put a sensor on a patient as they walk in the door.
As you think about what we're doing with Verily, our first goal with them is to make a disposable, low-cost transmitter as well. If you think about the hospital market, we could put a low-cost, disposable sensor on any patient with type 2 diabetes when they came in or even any other patient, with our Bluetooth capability, track it through the system. You have a wonderful means of measuring health. In cardiovascular wards, a very large percentage of those people have type 2 diabetes, and they spend a lot of time sticking fingers.
We are looking at that conversely with the 35% to 40% growth we're projecting next year and the over-50% growth we've had the past several years. We've had a hard time just taking care of all that we're doing, but I think that is a huge market for us in the future. I think gestational diabetes would be a huge market for this as well. We will look at other markets where glucose needs to be measured and have an influence on health outcomes.
Feroldi: Right. There's only so much that you do at one given time, so it makes sense. I know you said in your earnings call that now that you've launched Share, first off, you've seen tremendous patient demand. Second off, your phone's ringing off the hook essentially, with people having both questions about how to use it as well as probably just general iPhone questions.
Sayer: Yeah. We have learned a lot about people's iPhones. While a Dexcom receiver, all of them are exactly the same. I guarantee you if we took our phone into the lab and had our guys look at them, we would find they all do something different. Your email may download once every minute. Hers may download every two seconds. Mine may be every five minutes, and we've seen phones have emails that ask for a download every second, but when you're fighting a Wi-Fi radio like that for Bluetooth signal, you can see choppy results. We've learned a lot of intricacies about the iPhone to make sure that the data's all captured properly.
Feroldi: Is this going to repeat itself once you guys are available on the Android, which is obviously probably even more challenging?
Sayer: One of the reasons we've taken a longer time with Android is to make sure we understand it all. There are so many Android phones, we're going to have to pick some models and support them. We're not going to be able to go say, "This works on every Android phone," but we'll give a reasonable number of choices. We are learning.
Feroldi: If we could dig in a little bit to the Verily/Google [Alphabet (NASDAQ: GOOG)(NASDAQ: GOOGL)] partnership, obviously very exciting. I particularly like that you said that Dexcom has really talked to Google about your regulatory expertise, and they're teaching you about their electronic expertise, and it seems like a nice partnership.
Sayer: It's a wonderful partnership, and I'll go back to your background. Having worked in diabetes and in regulatory, you know how we always think in diabetes. Imagine being a cell phone manufacturer that throws away one model and launches a new one every six months. Those guys think really, really, really fast, and technology moves very, very fast for them. They have connections with suppliers, manufacturers, engineering talent that we don't have. As we met with them early on in the negotiations, they looked at us and said, "Everybody with diabetes should have this. You guys, we've got to make this grow."
They showed us their vision, and our joint vision is some day make that transmitter a Band-Aid that you throw away every time but make it cost effective to whereby we take cost out of the system, and we can still make money and everything works, but if CGM's a Band-Aid ... you've seen the size of the systems. Think how much less invasive that is in a child's life or a pregnant mom with gestational diabetes. If you're just wearing a Band-Aid on the back of your arm, nobody will even know what's going on. We think we can do that. That's the vision to make this that small and that easy to use.
They attack those electronic solutions, and they've made us think differently, "Well, yeah, we could do it that way, or we could do it this way." There's a lot of back-and-forth exchange of thoughts. Then, ultimately, when we get our data platform built... Dexcom, we're going to build our own platform to get the data, but there certainly will be Google Analytics capabilities and Verily analytics capabilities that can come into play with this. We'll start exploring that as well.
We're now just getting to the point with all the G5 Mobile patients to whereby we have a very substantial database of patient data flowing in every day. We've never had a bunch of data scientists sit down and run through, "OK, if we wanted to apply an algorithm to these 50,000 people, or how many, what can we learn? What is the general learning that we can learn and we can do here?" I think there's tremendous opportunity.
If you look out even a little further with it, that cloud data, when you have cloud algorithms or machine learning integrated up there talking back to patients, that's a lot easier than changing the physical form of a device from a regulatory process or running a new algorithm that you have to load on a transmitter or a phone or whatever. I think there's going to be some amazing changes and opportunities in diabetes over the next several years. It's going to be really remarkable.
Feroldi: I've got to ask. When you say "Band-Aid," you literally mean stick it on, no insertion, and somehow ...
Sayer: I mean Band-Aid. No, with our system, there will always be insertion because it's based on a wire, and right now, that wire inserts with a needle, but you can see the day where the needle goes away. We've tested needle-less sensors where maybe it's just a push-down through a form. We are working toward things like that to take costs down. I'm not sure how the Band-Aid will be inserted yet. That's a Dexcom task in the Google partnership, and that's one of our biggest variables is how are we going to do the insertion?
Any way you can take cost out is good, and to make a device where you have a needle and a spring and plastic, it's always going to be pretty expensive because it has to work 100% of the time for these people, for these patients. I think you could see a day where we have an insertion system without a needle.
Feroldi: That would be obviously very exciting and especially from a patient and provider training perspective.
Sayer: Wouldn't that be lovely?
Feroldi: Yeah. If you --
Sayer: One of the products that you may not be aware of, and we've talked about this on our calls, our next big launch, or our next big real physical milestone, is we have a new insertion system, a new transmitter, that we'll file in the first half of this year and we'd like to launch in the second half. Our next insertion system is a button push. Peel off the tape, put it on your skin, push the button, throw it out. That's all. It's all housed inside an egg-shaped insertion device to make it much, much easier for patients, and the transmitter profile is much lower than the current product.
Feroldi: Would that be your Gen 6 system?
Sayer: No, we'll do it on the Gen 5 system, and then Gen 6 will incorporate the same insertion technology. It's easier to make that physical change first with Gen 5, so we're only filing 1 change. Then, when we go to the next product, we file the next change, which is the Gen 6 sensor, which is a completely new sensor, with a different glucose computation algorithm.
Feroldi: You guys have always gotten more and more accurate. I assume that that's going to be a big initiative.
Sayer: There are two, there's really three features with Gen 6 that are very visible. Number 1, we're going to cut it down to a single calibration a day after start-up. Number 2, we are going to extend the life. Our goal is to extend the life to 10 days, because we know patients wear these longer than 10 days now anyway. We just as well have a 10-day life. The other very obvious physical feature is there's a blocking layer in the membranes that will block interference from other compounds. Our sensor now is labeled that if you take acetaminophen, there's an artificial rise. We're going to block all the drugs and eliminate that artificial noise.
Feroldi: That'd be great.
Sayer: There's a bunch of other very sophisticated scientific tweaks in the algorithm that'll just... you're right. We always go for more accuracy. We will go for more accuracy yet again, but we also want to give the patients an easier experience, and a single calibration a day through our research is very, very important. If all you got to do is wake up in the morning, stick your finger once, and go, and then imagine that with the dosing claim, boy, that's a good life. That's a good thing.
Feroldi: With the 10-day sensor, correct?
Sayer: Ten-day is what we're going to go for labeling.
Feroldi: What would that do to your revenue model?
Sayer: That's an interesting question, and there are two ways to look at it. The most optimistic business case for Dexcom obviously would be is we bill it, we get paid a certain amount per day from the payers, so you get paid that for the 10 days. My belief is we'll probably pick somewhere between seven and 10 and pass some cost savings onto payers and the patients as well. That's one of the [inaudible] we look at in the future as far as moving to pharmacy. With that 10-day sensor and increased pricing, maybe there are things we could work out with payers that'd get us to pharmacy quicker if we have a more per-day, more days of revenue model in the sensor.
Feroldi: That happened previously, correct? You guys increased --
Sayer: We went from three to seven.
Feroldi: Yep, and you guys --
Sayer: We got more, yeah.
Feroldi: Right. You increased it, which obviously does great things for margins.
Sayer: Yes, but when that happened, we were a $20 million company. Now that we're a $400 million to $600 million company, we're a little bigger deal, so I don't think the rubber stamp on going from approximately between 70 and 75 to 100 and 105, I don't think that'll be a rubber stamp. It's something we've got to run the study and get the product approved. We'll be very deliberate in our commercial strategy with respect to pricing on the product. One of the barriers is cost. It is for all these devices. To the extent we can take the cost out of the system for patients and still do well by our business, we're going to do that.
Feroldi: It seems like it's a win-win-win. It's a win for Dexcom, it's a win for patients, and it's a win for payers.
Sayer: A win for payers because, yeah, they get better outcomes, yeah.
Feroldi: Right, especially if you guys get that all-important replacement claim, which they can theoretically use even less strips over time.
Sayer: Fewer strips over time.
Feroldi: That's great.
Sayer: Theoretically, you'd only use the one or two used to calibrate, and that'd be it. You can take that cost out of the model.
Feroldi: If we could touch on your pump partnerships really fast. You guys have launched products with Johnson & Johnson (NYSE: JNJ)as well as Tandem Diabetes (NASDAQ: TNDM). Can you just let us know how that's going?
Sayer: In Europe, the Vibe [from Johnson & Johnson] has been very well accepted, and J&J has done very good with that product. I think they are growing in market share in every country where the Vibe has been launched. That's been a good European launch for us. They haven't been out in the U.S., only in '15, but I think there's pretty good traction so far, same with Tandem. They launched their Gen 4 integrated system in the fourth quarter.
The issue with both those systems is what I said earlier. They're Gen 4 integrated, and now patients can share and have Gen 5 data. We've been moving at a pace a little faster than our pump partners, and we've encouraged them to keep up. I would tell you --
Feroldi: You guys set the bar high.
Sayer: I tell you, over the past several months, we've seen all of them step up and go faster. They've all realized, I think, the value of the phone, that there can be apps that we can possible integrate and do things with.
We've also worked with some smaller pump companies. We have a partnership with Bigfoot, who's very visible in the diabetes community, the former Asante pump that's run by Bryan Mazlish and Jeffrey Brewer, who used to be the CEO over at JDRF. They're taking a very unique approach to what they want to do with the sensor-augmented system. I think the FDA's very warm to these systems. There's trials going on all over the world on this stuff, but it's all going to get back to cost and outcomes when all said and done.
I believe personally there's going to be a range of solutions for type 1 and intensive type 2 treatment that will start at the high end with those systems, but we're going to be able to give patients who don't want to wear a pump an incredible experience with the sensor. Then, as we start, we see software being developed by a number of companies that tell you what to do. The guys make fun of me. I keep telling them I want a Staples "Easy" button on our app that I'm sitting here at 99, what my thing says, and I'm about to eat breakfast, I want to hit a button that tells me what to do.
To the extent we can gather data from possibly an accelerometer or we can gather data from an insulin pump that computes insulin on board so we can have things similar to the bolus calculator used by the pump companies for pen patients. There are Bluetooth pens being developed. If we get every Bluetooth pen dose in our app, and we plan on filing an app later this year that would accept insulin information as well, we're going to offer a range of solutions for these people that will be very cost-effective.
Certainly, the sensor-augmented pump systems will be part of the solution, but I think the solution's much broader than that, but we all believe, we believe, obviously, that Dexcom CGM should be part of it for everybody.
Feroldi: Sure. You need the data to make those decisions.
Sayer: You need the data to make the decision. Meters have never been labeled for dosing. There is no device that's ever been labeled for dosing insulin. The reason that the authorities let meters be used in dosing is because they hold them to a very high accuracy standard. You do know, have a pretty good indication exactly what your blood sugar is to make a decision, but there's no labeling on a meter that says, "Based on this labeling, you can dose." This will be the first. The FDA's being very thoughtful. We're being very thoughtful. We want to make sure we do it right.
Feroldi: I've heard that the "artificial pancreas," it's going to be a couple of intermediate steps to get there.
Sayer: It'll be done in steps.
Feroldi: Obviously, one of the big first ones is just the replacement claim, which is the big step for you guys. What happens, what do you see as happening after that?
Sayer: The first step already happened with Medtronic's 530G pump that shut off insulin delivery if a pump was low, and as the FDA outlined the steps, that was the first step they wanted to happen. I think what you'll see then, as I look at our pump partners, you'll see things like a predictive shutoff. When we predict you're going to go low, they would shut it off. Johnson & Johnson talks about a hyper-hypo minimizer algorithm whereby if it sees you're going low, it would decrease insulin delivery. If it sees you're going high, it would increase insulin delivery based on an algorithm. I believe Tandem is doing work like that. I know Insuletis doing work like that as well.
It will happen in steps, and it will be interesting to see how far we go. I think the most useful application, in my own mind ... I'll be very simplistic. If you could just control them at night when they're sleeping, because during the day, if we're giving you a steady data feed and more information than you get now about, "Gee, you're low, you ought to do something," or, "Gee, you're high, you ought to do something," or, "Hey, did you just exercise? Because you seem to be going down real fast." When we can have interaction like that, that'll be wonderful, but while patients are sleeping, if these algorithms can really adjust insulin dosing and take care of patients, I think that would be a wonderful advance as well.
Again, where it gets interesting is going to be the cost and the benefit. You weigh the benefit of that thing that controls you at night and how much that's going to cost you versus our share system that wakes up your significant other that's there in the room with you or your parents in the room next door. You might get the same outcome. This is going to be an amazing time in diabetes. There's all these solutions coming to the front. How are the payers going to sort through it? What cases do we have to make to payers? How do we get it all approved so patients have the best outcome and a variety of choices?
Feroldi: Do you think that the agency's comfortable with having a device that would do as you say, minimize the basal rate or increase it, even if was just overnight?
Sayer: I think they're getting there, and I think the trials they're making these companies run are very extensive. I know it's a goal of the agencies and the understand it very well. Trials have been going on in this area for a long time. It's not like they don't have a data set. They do have a data set. Practical use will be different, so it'll be interesting to watch how big ... I got to believe there's going to be pretty large post-market studies for these things to make sure that they work properly. I think the agency's open to that, and they want to. We'll just have to see how it goes.
Feroldi: How has the medical device tax affected you guys?
Sayer: We sell directly to consumers, so the medical device tax has not had much of an effect on Dexcom. Our professional product we sell to physicians we pay medical device tax on, so we're in a nice place with respect to the medical device tax.
Feroldi: OK. If it was repealed, it wouldn't have much of an effect on your business, but you guys haven't been hurt on the revenue side?
Sayer: We've not been hurt like the other companies.
Feroldi: As you think about as your business scales, how do you prioritize showing profits on the bottom line versus continually reinvesting given all the opportunities that you guys have?
Sayer: As a former chief financial officer, this is the question I hope everybody always asks me because the numbers are where I'm always comfortable. Last year, we grew 55% at the top line, and our cash-based results more than doubled, but we didn't keep everything we added to the top line. We continued to reinvest. I think, given where we are as a company, that reinvestment's going to be very, very critical.
We're not competing against the Sisters of the Poor here. Medtronic and Abbott are large companies. They're going to spend, so for us to go into batten-down-the-hatches, generate-a-bunch-of-profits mode would be a horrible, horrible decision. The flip side of that is we need to show our shareholders that this business is leverage-able. We've attained 70% gross margins on our revenues -- 70% margin's pretty good.
Like I said, our cash-based operating income doubled from last year. In the fourth quarter, our cash-based net income was something like 22% of sales. That's when you back out all the stock compensation and some of the, like the charge for the Google technologies. When you back out all that stuff, that shows very good leverage in a quarter where we spent heavily on direct-to-consumer advertising. We continue to spend heavily on R&D, but the product pipeline has to keep coming.
If we don't continue to invest in R&D and get these new products out in studies, then the growth slows down, so it is a balancing act. Our overall goal is, other than extraordinary items, to grow the bottom line a lot faster than we grow the top while continuing to reinvest. We've been able to do that so far.
Now, in 2016, aside from our regular expense growth, we have 4 key initiatives that we're going to spend, and we've told investors, we give them very specific guidance, up to $40 million on our Verily partnership, our advanced data platform, our European expansion, and a new factory in Arizona, where we're going to build sensors.
We never thought we'd run out of capacity in San Diego, but as we look at the Verily partnership, we look at the pump partners all hitting their strides, we look at the type 2 operation, we look at a hospital market like you talked about, we decided it's imperative that we create some more capacity. Two years out when we might hit our max capacity in San Diego, we have another facility up and running.
We have four very strategic initiatives that we're spending on outside of the norm, but we'll still grow our cash-based results over the course of the year.
Feroldi: That's interesting that you're going to be investing in manufacturing in the United States versus, say, overseas. Is that because the process is so automated that the cost savings if you go overseas isn't worth it?
Sayer: We're looking to automate a lot of our processes, and our processes are so proprietary, we wanted to keep them here. One of the reasons we picked Arizona... we studied several geographies in the U.S. That's an hour plane ride from San Diego, so a lot of our executives can still supervise and be involved in that. As a growing company, we feel the need still to be very much in control. This isn't a cookie-cutter operation where you can go open a plant up in Mexico and it just goes. If building sensors were easy, there'd be a lot more companies in the business. It isn't, and so we need a level of control.
Feroldi: Funny you bring that up. On the competition front, I've personally been amazed that there hasn't been a flood of sensor companies that have come into the market, because the pump market has certainly been seeing new players, has seen very aggressive expansion. Why do you think that you guys essentially, in my mind, have the sensor market, essentially, it's been you and Medtronic, and that's it?
Sayer: It's hard. Honest to goodness, you can make sensors that work in a small clinical study, and then when you've got to make millions of them, it is not an easy business. A lot of the technologies have come and gone, or companies start up, but they don't see a way to scale. It's tough. The demands of these patients, this thing's got to be good. Your life depends on it. There are a lot of start-up sensor companies now, and we look at all of them. If anything interests us, we take a look, but so far, we're very happy with our own technology.
Commercializing it is different. It's like the pump companies. You come from the pump world. Our head R&D guy at MiniMed when I was there used to say something real interesting, "It costs quite a bit to make that pump, but it costs a whole lot more to put the 1-800 number on the back of it." That's the learning that all these start-up companies get to.
Feroldi: Takes a heck of a lot of capital to get over that hump and scale the organization. Essentially, manufacturing, customer support, are essentially barriers to entry.
Sayer: They are, in addition to technology, both of them.
Feroldi: Quickly, if we could touch on, you mentioned that you launched a direct-to-consumer campaign that was in print, and you guys seem to drive record visits to your website, record impressions. I'm sure your phone was ringing off the hook. You obviously saw a lot of success with that, and you said that you're going to continue that?
Sayer: We are.
Feroldi: Indefinitely, or just as long as it makes sense?
Sayer: Certainly for the first half of this year, and we'll continue what we did in the fourth quarter or the first half of this year with the G5 Mobile. Then, we'll re-evaluate, but I think the days of not going to consumers are over. I think we need to go directly to patients.
I'll give you the perfect example. I was talking with somebody who was very close to me in their 40s just diagnosed with type 1. I showed them my CGM. Never heard of it, never seen it. Knows me very well. "This is what you do?" "Yeah." "Why hasn't my doctor told me about this?" "Don't know, but you should go talk to your physician and get on it." There's still awareness hurdles that we just got to overcome.
I'm a patient on the caregiver side, but in most cases, when you walk into your caregiver and say, "I want that," they're pretty open to it, particularly those that understand CGM. Now, why they don't offer it to everybody that walks in the door, that would be our dream. It's just not there yet with any device or any drug, but to the extent we can make more progress with awareness with patients and providers, that's where our campaigns will go this year on both fronts.
Feroldi: What kind of penetration rates do you think CGM has in, say, into the U.S. type 1 market? There's about 1,500,000 or so type 1s.
Sayer: You look at the U.S. market as 1,500,000. We've said our total patient base is somewhere around 140,000 to 150,000 with 20%, 25% of that overseas, so you can extrapolate us. We're somewhere above 100,000 patients in the U.S. Between us and Medtronic, I think it's somewhere in the 15% to 20%, but 10% to 20% range certainly. It's come up a lot. We also said on a call we nearly doubled our patient base last year. It was a busy year, so penetration really started to spike last year.
I'm not quite sure why a type 1 wouldn't want to wear a sensor, having experience. The data is just so rich and so valuable in your life. That's why we're coming at this from a variety of different solutions. With phone connectivity, with the sharing, decreasing the footprint and the size and all the things I talked about earlier will just appeal to more people. How high can penetration get? Gosh, I'd be very disappointed if we didn't get to 70% to 80% penetration in the type 1 market for CGM over time.
Feroldi: Theoretically, if payers see data and they see cost savings, they'll lower the barriers, they'll make it more accessible.
Sayer: They'll do that. You know what? That's our job, too. I don't think we've done a perfect job of laying out the cost of CGM versus the benefit of CGM. I think, over time, particular gathering this data in the cloud from our patients, we're going to be able to gather some certainly better outcome data, and the insurance companies can track the cost data. That outcome data will become evident on the next few earnings calls.
Feroldi: Again, your growth is especially impressive considering that the trend over the last two or three years is really to push more of the cost of care directly to the patient. We saw deductibles rise. We've seen patients with $5,000 deductibles, so when you --
Sayer: It's awful. The phone calls are awful. The emails are awful. "My daughter has type 1. They raised my deductible to $10,000. I can't afford CGM. What can you do for me?" Responding back to those emails is the worst part of my day. The other bad emails, in all honesty, are the 64-year-old people who are going to turn 65 and, "I'm not going to be eligible for your device next week. What can you do?" We need to fix that.
Feroldi: Right. Hopefully, that's something that fixes itself over time as you made the case to payers.
Sayer: Over time. You bet.
The article The Motley Fool Sits Down With the CEO of Dexcom Inc. originally appeared on Fool.com.
Suzanne Frey, an executive at Alphabet, is a member of The Motley Fool's board of directors. Brian Feroldi owns shares of Alphabet (A shares), Alphabet (C shares), and Insulet. The Motley Fool owns shares of and recommends Alphabet (A shares) and Alphabet (C shares). The Motley Fool owns shares of Medtronic. The Motley Fool recommends Insulet and Johnson & Johnson. Try any of our Foolish newsletter services free for 30 days. We Fools may not all hold the same opinions, but we all believe that considering a diverse range of insights makes us better investors. The Motley Fool has a disclosure policy.
Copyright 1995 - 2016 The Motley Fool, LLC. All rights reserved. The Motley Fool has a disclosure policy.